推荐产品
化驗
>95% (HPLC)
形狀
solid or viscous liquid
反應適用性
reaction type: Biotinylations
reaction type: Pegylations
聚合物結構
shape: linear
functionality: monofunctional
運輸包裝
ambient
儲存溫度
−20°C
特點和優勢
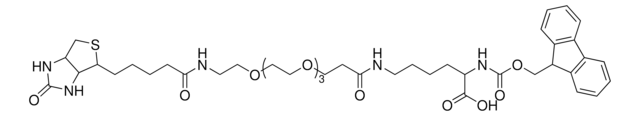
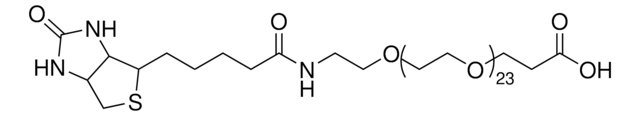
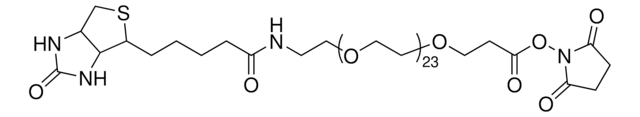
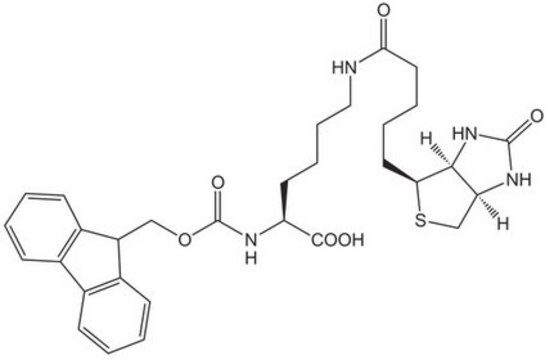
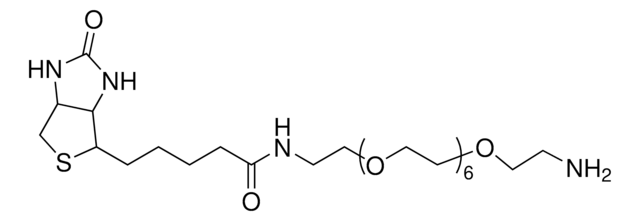
dPEG4 biotin acid consists of biotin and a propionic acid-functionalized tetraethylene glycol derivative. Numerous functional or reactive groups can modify the terminal acid moiety, including the acylating agents N-hydroxysuccinimide (NHS) and 2,3,5,6-tetrafluorophenol (TFP). Also, dPEG4 biotin acid reacts directly with primary amines using a suitable carbodiimide such as 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC). The amphiphilic dPEG linker gives biotin excellent solubility in water and aqueous buffer, where biotin usually is poorly soluble. dPEG4 biotin acid offers significantly superior solubility and performance characteristics compared to the traditional, highly hydrophobic biotinylation reagent known as LC-biotin. Moreover, although dPEG4 biotin acid and LC-biotin have comparable linker lengths, dPEG4 biotin acid will not trigger the aggregation and precipitation of conjugated biomolecules, even at high levels of biotin incorporation on the biomolecule. In contrast, LC-biotin triggers the aggregation and precipitation of biomolecules with the incorporation of a few LC-biotin groups onto the biomolecule.
Automate your Biotin tagging with Synple Automated Synthesis Platform (SYNPLE-SC002)
Automate your Biotin tagging with Synple Automated Synthesis Platform (SYNPLE-SC002)
法律資訊
Products Protected under U.S. Patent #s 7,888,536 & 8,637,711 and European Patent #s 1,594,440 & 2,750,681
dPEG is a registered trademark of Quanta BioDesign
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Alexander Kuzmin et al.
Bioconjugate chemistry, 21(11), 2076-2085 (2010-10-23)
The utility of catalyst-free azide-alkyne [3 + 2] cycloaddition for the immobilization of a variety of molecules onto a solid surface and microbeads was demonstrated. In this process, the surfaces are derivatized with aza-dibenzocyclooctyne (ADIBO) for the immobilization of azide-tagged
Global Trade Item Number
| 货号 | GTIN |
|---|---|
| QBD10199-100MG | 4061842703159 |
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持
![O-[2-(生物素基-氨基)乙基]-O′-(2-羧乙基)聚乙二醇 3000 Mp 3,000](/deepweb/assets/sigmaaldrich/product/structures/285/242/a1e6e88b-5b7d-43b5-9bb0-18dcbfdccf43/640/a1e6e88b-5b7d-43b5-9bb0-18dcbfdccf43.png)
![N-[2-[2-[2-(2-叠氮乙氧基)乙氧基]乙氧基]乙基]生物素胺](/deepweb/assets/sigmaaldrich/product/structures/120/306/c9779b03-3754-4ad6-8eef-b07209e113ce/640/c9779b03-3754-4ad6-8eef-b07209e113ce.png)