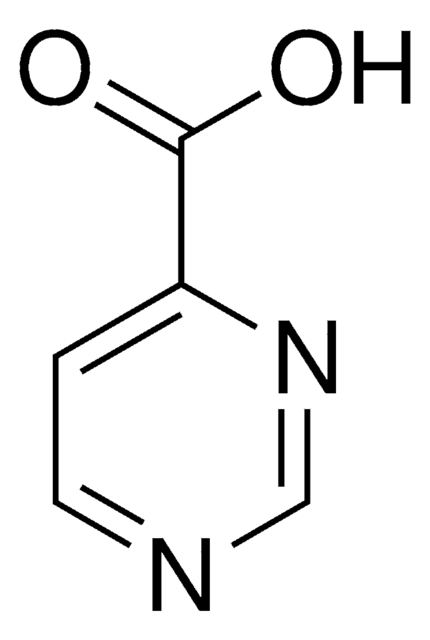
推荐产品
化驗
99%
形狀
powder
mp
222-225 °C (dec.) (lit.)
SMILES 字串
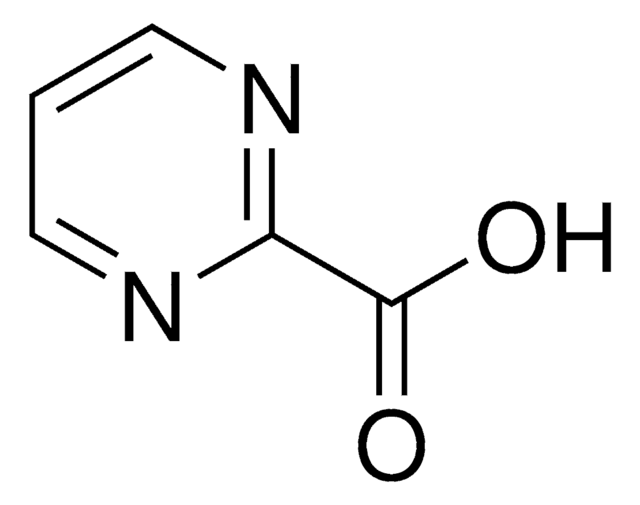
OC(=O)c1cnccn1
InChI
1S/C5H4N2O2/c8-5(9)4-3-6-1-2-7-4/h1-3H,(H,8,9)
InChI 密鑰
NIPZZXUFJPQHNH-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
Mohamed Abdel-Aziz et al.
European journal of medicinal chemistry, 45(8), 3384-3388 (2010-05-22)
A series of pyrazine-2-carboxylic acid hydrazide derivatives were synthesized and screened for their activity against Mycobacterium tuberculosis. The results show that pyrazine-2-carboxylic acid hydrazide-hydrazone derivatives 3a-l were less active than pyrazinamide. In contrast, the N(4)-ethyl-N(1)-pyrazinoyl-thiosemicarbazide 4 showed the highest activity
Rustam Z Khaliullin et al.
The journal of physical chemistry. B, 109(38), 17984-17992 (2006-07-21)
Experimental studies by Shul'pin and co-workers have shown that vanadate anions in combination with pyrazine-2-carboxylic acid (PCA identical with pcaH) produce an exceptionally active complex that promotes the oxidation of alkanes and other organic molecules. Reaction of this complex with
Wolfgang Holzer et al.
Magnetic resonance in chemistry : MRC, 47(7), 617-624 (2009-04-30)
NMR spectroscopic studies are undertaken with derivatives of 2-pyrazinecarboxylic acid. Complete and unambiguous assignment of chemical shifts ((1)H, (13)C, (15)N) and coupling constants ((1)H,(1)H; (13)C,(1)H; (15)N,(1)H) is achieved by combined application of various 1D and 2D NMR spectroscopic techniques. Unequivocal
Ping Lu et al.
Antimicrobial agents and chemotherapy, 55(11), 5354-5357 (2011-08-31)
Pyrazinoic acid, the active form of the first-line antituberculosis drug pyrazinamide, decreased the proton motive force and respiratory ATP synthesis rates in subcellular mycobacterial membrane assays. Pyrazinoic acid also significantly lowered cellular ATP levels in Mycobacterium bovis BCG. These results
Mirko Zimic et al.
Tuberculosis (Edinburgh, Scotland), 92(1), 84-91 (2011-10-19)
Pyrazinamide is one of the most important drugs in the treatment of latent Mycobacterium tuberculosis infection. The emergence of strains resistant to pyrazinamide represents an important public health problem, as both first- and second-line treatment regimens include pyrazinamide. The accepted
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门