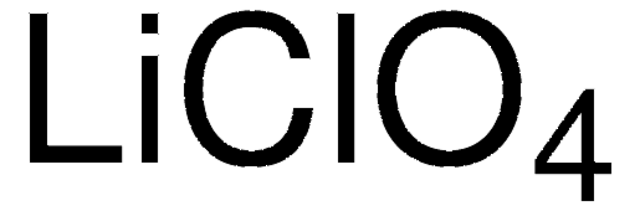推荐产品
等級
anhydrous
battery grade
品質等級
化驗
≥99.9% trace metals basis
形狀
powder
環保替代產品特色
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
雜質
≤1000 ppm (trace metals analysis)
pH值
6.0-7.5 (25 °C, 5%, aq.sol.)
mp
236 °C (lit.)
溶解度
H2O: 59.8 g/dL at 25 °C
負離子痕跡
chloride (Cl-): ≤30 ppm
sulfate (SO42-): ≤10 ppm
正離子痕跡
Fe: ≤5 ppm
heavy metals: ≤10 ppm
應用
battery manufacturing
環保替代類別
SMILES 字串
[Li+].[O-]Cl(=O)(=O)=O
InChI
1S/ClHO4.Li/c2-1(3,4)5;/h(H,2,3,4,5);/q;+1/p-1
InChI 密鑰
MHCFAGZWMAWTNR-UHFFFAOYSA-M
正在寻找类似产品? 访问 产品对比指南
一般說明
Anhydrous lithium perchlorate is a white-to-colorless crystalline salt. It is hygroscopic and deliquescent and usually stored under inert atmosphere. It is highly soluble in water and soluble in a variety of organic solvents including alcohols, acetone, acetonitrile, ethyl acetate, ethers, carbonates, and other polar organic solvents. Lithium perchlorate is a strong oxidizing agent.
Industrially, lithium perchlorate is manufactured in several ways. Most commonly, it is prepared from sodium perchlorate through a metathesis reaction with lithium chloride or lithium carbonate. Lithium perchlorate can also be prepared by direct electrochemical oxidation of lithium chloride or by reacting lithium carbonate with perchloric acid. The hydrate can be dried either by highly controlled heating or by displacing water with volatile amines, which are removed by drying under vacuum.
Industrially, lithium perchlorate is manufactured in several ways. Most commonly, it is prepared from sodium perchlorate through a metathesis reaction with lithium chloride or lithium carbonate. Lithium perchlorate can also be prepared by direct electrochemical oxidation of lithium chloride or by reacting lithium carbonate with perchloric acid. The hydrate can be dried either by highly controlled heating or by displacing water with volatile amines, which are removed by drying under vacuum.
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for energy efficiency. Click here for more information.
應用
The primary application of lithium perchlorate is as an electrolytic salt in lithium-ion batteries. Many of the early, now-famous reports of lithium batteries used lithium perchlorate dissolved in polar organics as the electrolyte and the salt remains popular because of its high solubility, electrochemical stability, and low cost. In the search for solid electrolytes, lithium perchlorate (5-12 wt%) is often added to polyethylene oxide (PEO) and composited with ceramic nanoparticles like LLZO and LATP .
Researchers also use lithium perchlorate as an electrolytic salt in aqueous media when testing electrocatalysts. For example, recent experiments improving the electrochemical reduction of nitrogen over TiO2 nanoparticles or gold nanoparticles use aqueous lithium perchlorate as the electrolyte.
Researchers also use lithium perchlorate as an electrolytic salt in aqueous media when testing electrocatalysts. For example, recent experiments improving the electrochemical reduction of nitrogen over TiO2 nanoparticles or gold nanoparticles use aqueous lithium perchlorate as the electrolyte.
包裝
100g in poly bottle
500g in poly bottle
500g in poly bottle
訊號詞
Danger
危險分類
Acute Tox. 4 Oral - Eye Dam. 1 - Ox. Sol. 2 - Skin Corr. 1A - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
5.1A - Strongly oxidizing hazardous materials
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Electrochemical and In Situ X?Ray Diffraction Studies of Lithium Intercalation in Lix CoO2
Reimers, J.N., et al.
Journal of the Electrochemical Society, 139, 2091-2091 (1992)
The spinel phase of lithium manganese oxide (LiMn2O4) as a cathode in secondary lithium cells
Tarascon, J.M., et al.
Journal of the Electrochemical Society, 138, 2859-2864 (1991)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门