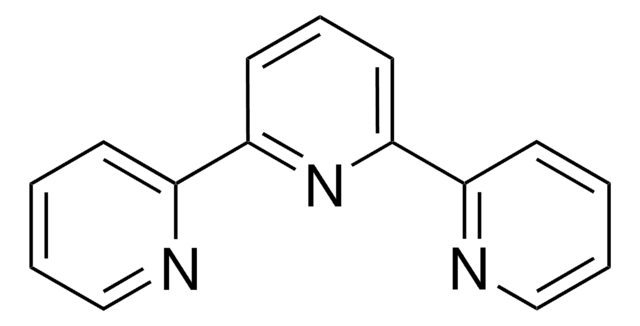
推荐产品
化驗
≥95%
形狀
powder or crystals
反應適用性
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
mp
>300 °C
儲存溫度
2-8°C
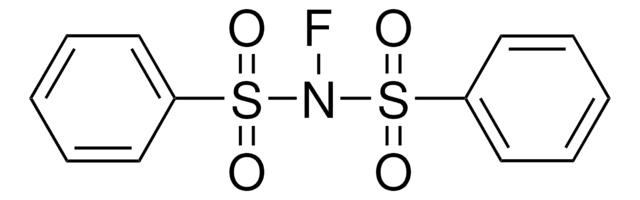
SMILES 字串
N#CC.C1(C2=CC=CC=N2)=CC=CC(C3=NC=CC=C3)=N1.[Pd]
InChI
1S/C15H11N3.C2H3N.Pd/c1-3-10-16-12(6-1)14-8-5-9-15(18-14)13-7-2-4-11-17-13;1-2-3;/h1-11H;1H3;
InChI 密鑰
UESWWXSSNHGEKT-UHFFFAOYSA-N
相关类别
應用
[Pd(terpy)(MeCN)][BF4]2 is a versatile palladium precatalyst.
Automate your fluorination reactions with Synple Automated Synthesis Platform (SYNPLE-SC002)
Automate your fluorination reactions with Synple Automated Synthesis Platform (SYNPLE-SC002)
相關產品
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Anthony R Mazzotti et al.
Journal of the American Chemical Society, 135(38), 14012-14015 (2013-09-18)
A practical, palladium-catalyzed synthesis of aryl fluorides from arylboronic acid derivatives is presented. The reaction is operationally simple and amenable to multigram-scale synthesis. Evaluation of the reaction mechanism suggests a single-electron-transfer pathway, involving a Pd(III) intermediate that has been isolated
Kumiko Yamamoto et al.
Nature, 554(7693), 511-514 (2018-02-23)
Aryl fluorides are widely used in the pharmaceutical and agrochemical industries, and recent advances have enabled their synthesis through the conversion of various functional groups. However, there is a lack of general methods for direct aromatic carbon-hydrogen (C-H) fluorination. Conventional
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门
![(2R,2′R-(+)-[N,N′-双(2-吡啶基甲基)]-2,2′-联吡咯烷双(乙腈)六氟锑酸铁(II)](/deepweb/assets/sigmaaldrich/product/structures/202/913/41951272-e38c-4f7b-9bf9-6ed9448f4864/640/41951272-e38c-4f7b-9bf9-6ed9448f4864.png)

2 ≥95%](/deepweb/assets/sigmaaldrich/product/structures/276/288/a8ff851d-d6be-43a1-b43f-1438e7b840d7/640/a8ff851d-d6be-43a1-b43f-1438e7b840d7.png)