推荐产品
化驗
95%
形狀
powder
mp
170.5 °C
應用
peptide synthesis
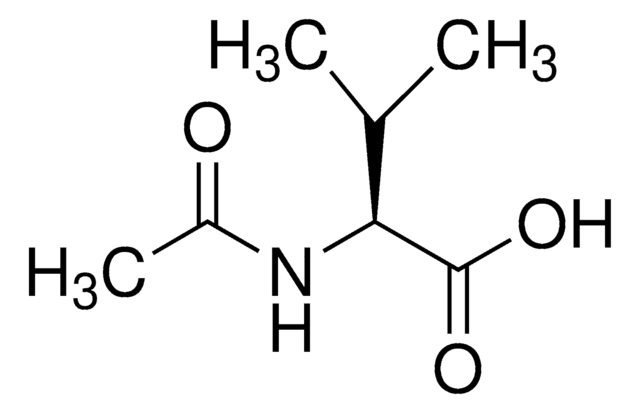
SMILES 字串
O=C(O)[C@H](C(C)C)NC(C)=O
InChI
1S/C7H13NO3/c1-4(2)6(7(10)11)8-5(3)9/h4,6H,1-3H3,(H,8,9)(H,10,11)/t6-/m0/s1
InChI 密鑰
IHYJTAOFMMMOPX-LURJTMIESA-N
一般說明
Ac-Val-OH is an N-protected valine amino acid ligand. It participates in the 2,6-diolefination reaction of phenylacetic acids.
應用
Ac-Val-OH may be employed as a ligand for the meta-selective tert-alkylation reaction.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Jie Li et al.
Journal of the American Chemical Society, 137(43), 13894-13901 (2015-09-30)
Acylated amino acid ligands enabled ruthenium(II)-catalyzed C-H functionalizations with excellent levels of meta-selectivity. The outstanding catalytic activity of the ruthenium(II) complexes derived from monoprotected amino acids (MPAA) set the stage for the first ruthenium-catalyzed meta-functionalizations with removable directing groups. Thereby
Keary M Engle et al.
Journal of the American Chemical Society, 132(40), 14137-14151 (2010-09-22)
Initial rate studies have revealed dramatic acceleration in aerobic Pd(II)-catalyzed C-H olefination reactions of phenylacetic acids when mono-N-protected amino acids are used as ligands. In light of these findings, systematic ligand tuning was undertaken, which has resulted in drastic improvements
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门

![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)二氯甲烷络合物](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)