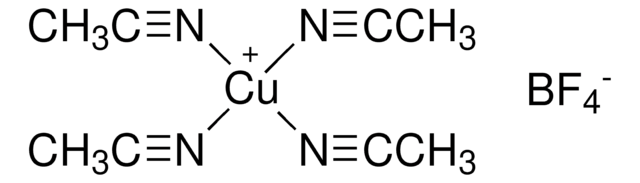
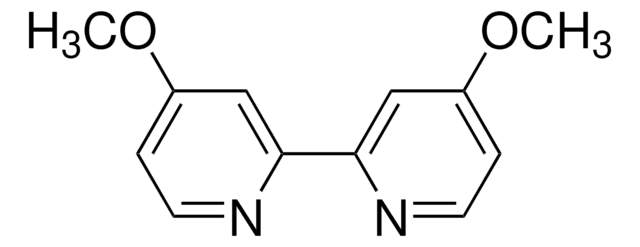
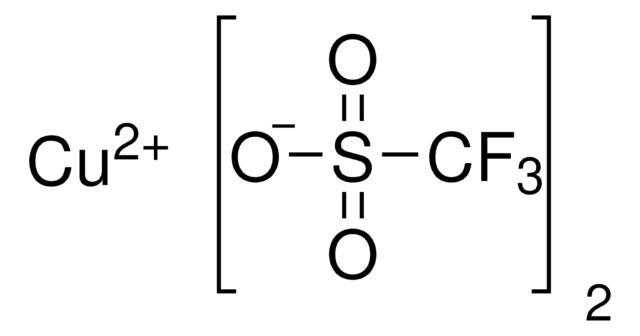推荐产品
品質等級
形狀
liquid
反應適用性
reagent type: oxidant
濃度
0.2 M in acetonitrile
儲存溫度
2-8°C
SMILES 字串
CN1C=CN=C1.CC2(C)CCCC(C)(C)N2[O].C3(C4=NC=CC=C4)=NC=CC=C3
InChI
1S/C10H8N2.C9H18NO.C4H6N2/c1-3-7-11-9(5-1)10-6-2-4-8-12-10;1-8(2)6-5-7-9(3,4)10(8)11;1-6-3-2-5-4-6/h1-8H;5-7H2,1-4H3;2-4H,1H3
InChI 密鑰
BQFURWVGIDXRNB-UHFFFAOYSA-N
一般說明
應用
訊號詞
Danger
危險分類
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 2 - Repr. 2 - Skin Corr. 1C
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
35.6 °F
閃點(°C)
2.0 °C
其他客户在看
商品
Alcohol oxidation is one of the most frequently performed oxidation reactions in organic chemistry. The aldehyde and ketone products of alcohol oxidation are useful intermediates en route to complex molecules.
Alcohol oxidation is one of the most frequently performed oxidation reactions in organic chemistry. The aldehyde and ketone products of alcohol oxidation are useful intermediates en route to complex molecules.
TEMPO (2,2,6,6-Tetramethylpiperidinyloxy or 2,2,6,6-Tetramethylpiperidine 1-oxyl) and its derivatives are stable nitroxy radicals used as catalysts in organic oxidation reactions. TEMPO was discovered by Lebedev and Kazarnovskii in 1960. The stable free radical nature of TEMPO is due to the presence of bulky substituent groups, which hinder the reaction of the free radical with other molecules.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门
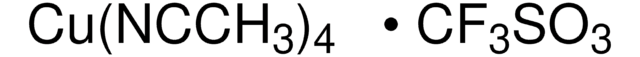
![9-氮杂双环[3.3.1]壬烷N-氧基 95%](/deepweb/assets/sigmaaldrich/product/structures/287/155/e2f4a2e1-1d4e-4bed-9187-9e16d23cbbbf/640/e2f4a2e1-1d4e-4bed-9187-9e16d23cbbbf.png)

![1,8-二氮杂双环[5.4.0]十一碳-7-烯 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)