About This Item
推荐产品
形狀
solid
品質等級
特點
generation 2
反應適用性
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
reaction type: Cross Couplings
mp
167-170 °C (decomposition)
官能基
phosphine
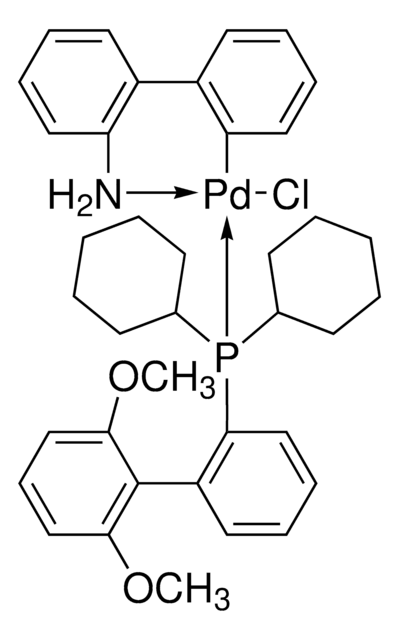
SMILES 字串
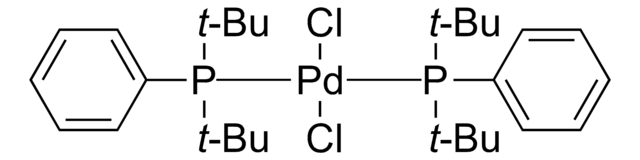
NC1=C(C2=CC=CC=C2[Pd]Cl)C=CC=C1.CC(C)(C)P(C(C)(C)C)C(C)(C)C
InChI
1S/C12H10N.C12H27P.ClH.Pd/c13-12-9-5-4-8-11(12)10-6-2-1-3-7-10;1-10(2,3)13(11(4,5)6)12(7,8)9;;/h1-6,8-9H,13H2;1-9H3;1H;/q;;;+1/p-1
InChI 密鑰
ZVSLIOFJVMRWHJ-UHFFFAOYSA-M
相关类别
一般說明
應用
- 通过芳基氯化物的交叉偶联反应,进行空间位阻联芳基(四邻位-取代)的合成。
- 芳基氯化物的Stille交叉偶联反应。
- 通过Stille交叉偶联反应合成氯代肽I。
- Heck反应。
- Negishi交叉偶联反应。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
商品
All of the preformed catalysts used in the kit are air and moisture stable complexes in their commercially available form. Once activated by base under the reaction conditions they become sensitive to air. To best enable scale-up success, the use of standard Schlenk technique is recommended.
All of the preformed catalysts used in the kit are air and moisture stable complexes in their commercially available form. Once activated by base under the reaction conditions they become sensitive to air. To best enable scale-up success, the use of standard Schlenk technique is recommended.
All of the preformed catalysts used in the kit are air and moisture stable complexes in their commercially available form. Once activated by base under the reaction conditions they become sensitive to air. To best enable scale-up success, the use of standard Schlenk technique is recommended.
All contents in the foil bag are weighed, plated, packed, and sealed in a glove box under nitrogen.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门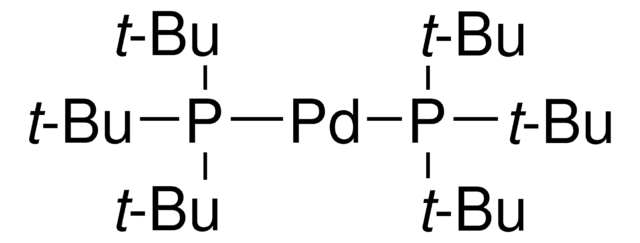
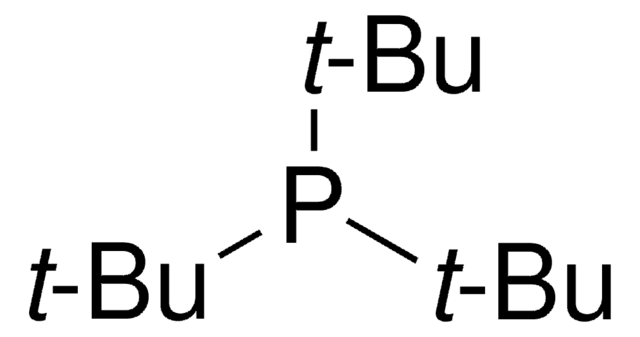
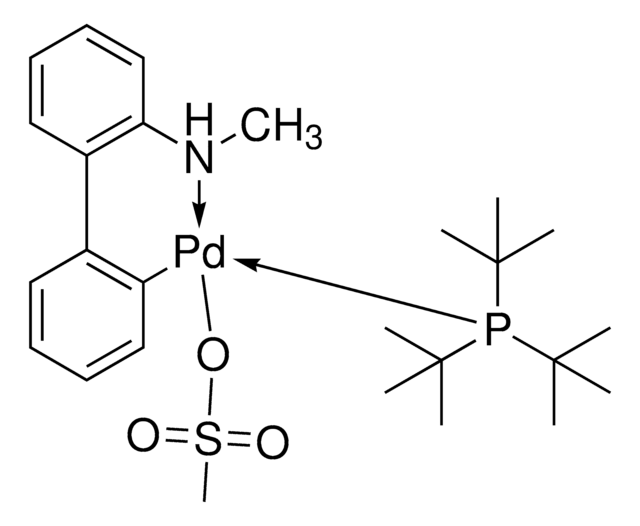
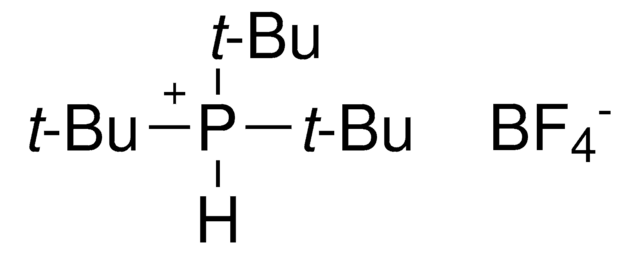
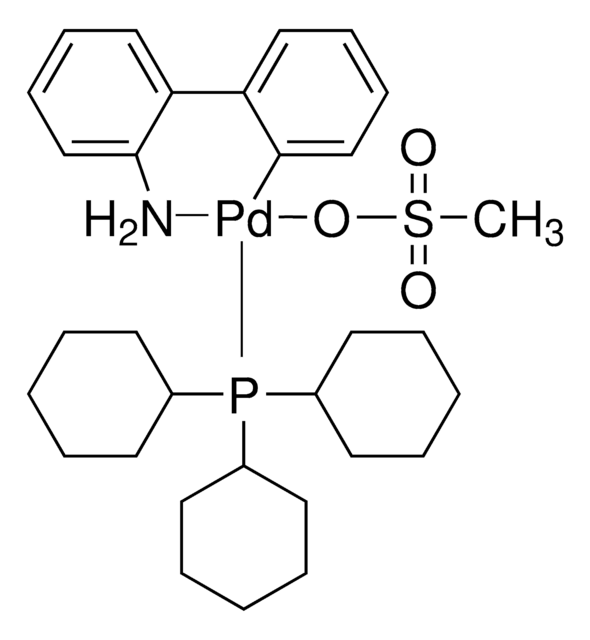
![Mesyl[(tri-t-butylphosphine)-2-(2-aminobiphenyl)]palladium(II)](/deepweb/assets/sigmaaldrich/product/structures/358/298/6539c19e-808c-4cd1-b9e8-19c6928f2384/640/6539c19e-808c-4cd1-b9e8-19c6928f2384.png)

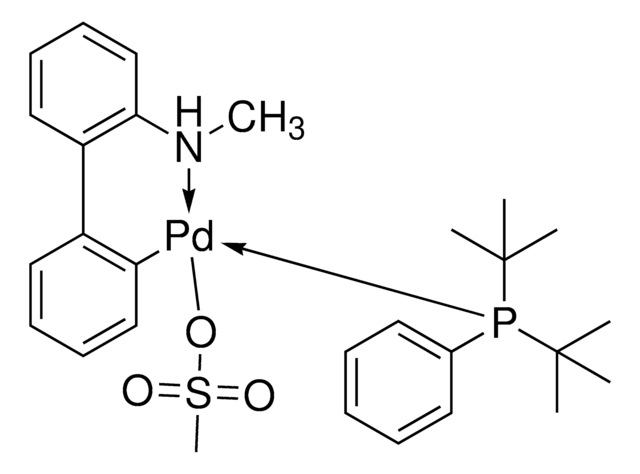
![[(二(1-金刚烷基)丁基膦基)-2-(2′-氨基-1,1′-联苯基)]钯(II)甲磺酸酯 95%](/deepweb/assets/sigmaaldrich/product/structures/391/492/af15708b-9501-44ae-a25f-d3574589a865/640/af15708b-9501-44ae-a25f-d3574589a865.png)