About This Item
推荐产品
形狀
solid
品質等級
反應適用性
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Cross Couplings
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
環保替代產品特色
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
258-272 °C
環保替代類別
, Aligned
儲存溫度
−20°C
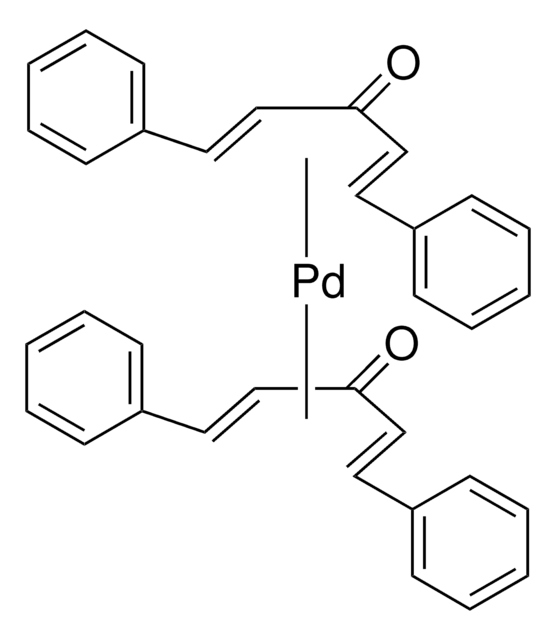
SMILES 字串
[Pd].CC(C)(C)P(C(C)(C)C)C(C)(C)C.CC(C)(C)P(C(C)(C)C)C(C)(C)C
InChI
1S/2C12H27P.Pd/c2*1-10(2,3)13(11(4,5)6)12(7,8)9;/h2*1-9H3;
InChI 密鑰
MXQOYLRVSVOCQT-UHFFFAOYSA-N
相关类别
一般說明
應用
- 多取代sp3-碳Suzuki偶联反应的催化剂(eq. 1)
- 氯代芳烃Stille偶联反应的催化剂(等式2)
- Negishi偶联反应的催化剂(等式3)
- Heck偶联反应生成四取代烯烃的催化剂(等式4)
- 卤代芳烃Buchwald-Hartwig胺化的催化剂 (等式5)
- 卤代芳烃与氨甲酰基硅烷发生羰基化反应的催化剂(等式6)
On the Way Towards Greener Transition-Metal-Catalyzed Processes as Quantified by E Factors
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
商品
TPGS-750-M, a second generation surfactant, is useful for room temperature, palladium and ruthenium-catalyzed reactions in water. Reactions include the Heck reaction, Suzuki-Miyaura reaction, Sonogashira reaction, Buchwald-Hartwig amination reaction, Negishi reaction, and olefin metathesis.
TPGS-750-M, a second generation surfactant, is useful for room temperature, palladium and ruthenium-catalyzed reactions in water. Reactions include the Heck reaction, Suzuki-Miyaura reaction, Sonogashira reaction, Buchwald-Hartwig amination reaction, Negishi reaction, and olefin metathesis.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-双(二-叔丁基膦基)二茂铁]二氯合钯(II) 98%](/deepweb/assets/sigmaaldrich/product/structures/192/459/02d1239c-1119-49d9-b392-a04d8f53855c/640/02d1239c-1119-49d9-b392-a04d8f53855c.png)

![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)二氯甲烷络合物](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)
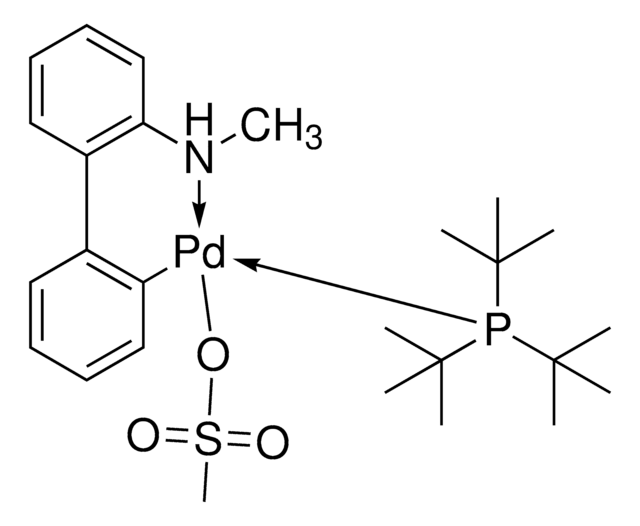
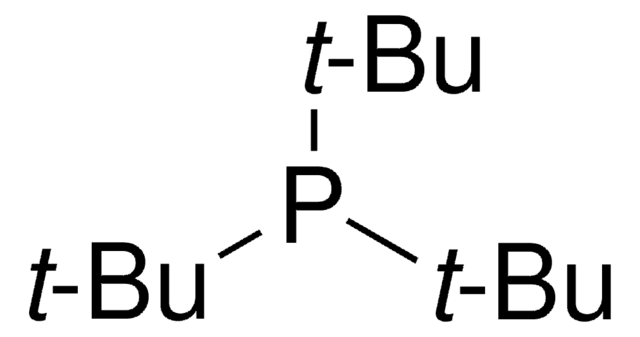

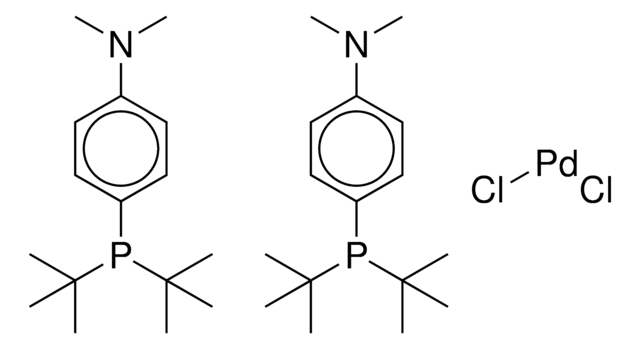
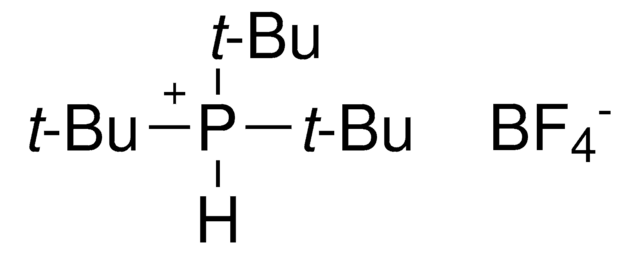
![氯[(三-TERT-三丁基膦)-2-(2-氨基联苯)]钯(II)](/deepweb/assets/sigmaaldrich/product/structures/100/957/42c5dad6-6197-4fa6-8481-3fe55f0291e9/640/42c5dad6-6197-4fa6-8481-3fe55f0291e9.png)
![Mesyl[(tri-t-butylphosphine)-2-(2-aminobiphenyl)]palladium(II)](/deepweb/assets/sigmaaldrich/product/structures/358/298/6539c19e-808c-4cd1-b9e8-19c6928f2384/640/6539c19e-808c-4cd1-b9e8-19c6928f2384.png)