所有图片(1)
About This Item
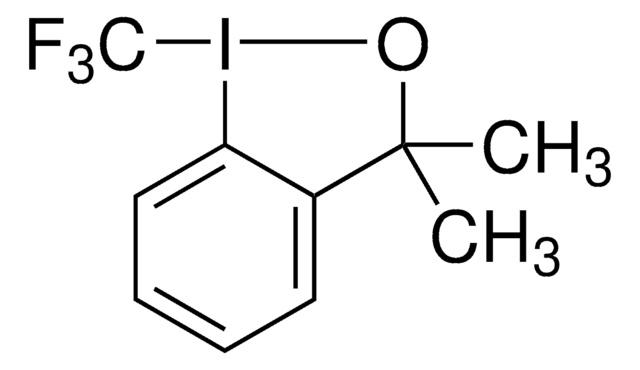
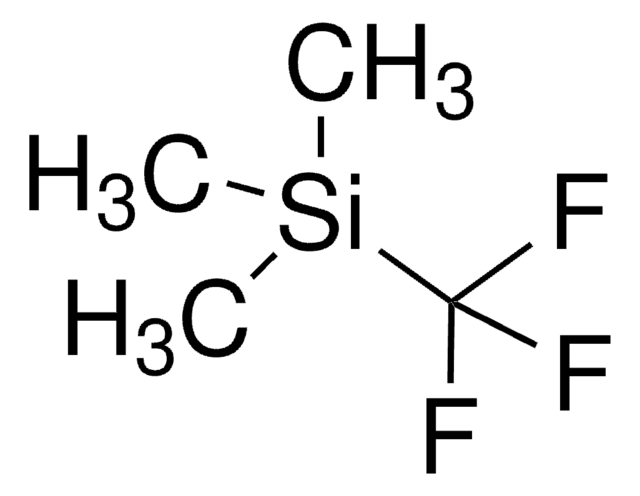
经验公式(希尔记法):
C10H10F3IO
CAS号:
分子量:
330.09
MDL號碼:
分類程式碼代碼:
12352101
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
98%
形狀
powder
mp
75-79 °C
儲存溫度
2-8°C
SMILES 字串
CC1(C)O[I](c2ccccc12)C(F)(F)F
InChI
1S/C10H10F3IO/c1-9(2)7-5-3-4-6-8(7)14(15-9)10(11,12)13/h3-6H,1-2H3
InChI 密鑰
HVAPLSNCVYXFDQ-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
- Selective trifluoromethylation of 1,3-disubstituted arenes through iridium-catalyzed arene borylation and copper-catalyzed trifluoromethylation
- Copper-catalyzed trifluoromethylation of aryl- and alkenylboronic acids with electrophilic trifluoromethylating reagent
- Pd-catalyzed electrophilic ortho-trifluoromethylation of arenes using trifluoroacetic acid as a promotor
Used in the Preparation of
- Trifluoromethylimidoylethyl substituted heterocycles via bis(trifluoromethylsulfonyl)amine-catalyzed Rotter type reaction of heterocycles with nitriles in presence of trifluoromethylbenziodoxole
- Stereoselective synthesis of α-trifluoromethyl aldehydes via trimethylbenzylimidazolidinone and copper-catalyzed enantioselective α-trifluoromethylation of aldehydes with iodonium salts
訊號詞
Warning
危險分類
Eye Irrit. 2 - Flam. Sol. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
4.1B - Flammable solid hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Tianfei Liu et al.
Angewandte Chemie (International ed. in English), 51(2), 540-543 (2011-12-01)
The old one two: A sequential iridium-catalyzed borylation and copper-catalyzed trifluoromethylation of arenes is described (see scheme; Pin = pinacol). The reaction is conducted under mild reaction conditions and tolerates a variety of functional groups. The advantages of this tandem
Tianfei Liu et al.
Organic letters, 13(9), 2342-2345 (2011-04-09)
A copper-catalyzed trifluoromethylation of aryl- and alkenylboronic acids with Togni's reagent was described. The reaction proceeded in good to excellent yields for a range of different substrates including heteroarylboronic acids and substrates with a variety of functional groups under mild
Anna E Allen et al.
Journal of the American Chemical Society, 132(14), 4986-4987 (2010-03-20)
An enantioselective organocatalytic alpha-trifluoromethylation of aldehydes has been accomplished using a commercially available, electrophilic trifluoromethyl source. The merging of Lewis acid and organocatalysis provides a new strategy for the enantioselective construction of trifluoromethyl stereogenicity, an important chiral synthon for pharmaceutical
Xisheng Wang et al.
Journal of the American Chemical Society, 132(11), 3648-3649 (2010-02-27)
A Pd(II)-catalyzed C-H activation/trifluoromethylation of arenes with an electrophilic trifluoromethylation reagent using diverse heterocycle directing groups is reported. The presence of trifluoroacetic acid is crucial for this catalytic reaction.
A Ritter-type reaction: direct electrophilic trifluoromethylation at nitrogen atoms using hypervalent iodine reagents.
Katrin Niedermann et al.
Angewandte Chemie (International ed. in English), 50(5), 1059-1063 (2011-01-27)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门