推荐产品
品質等級
化驗
98%
光學活性
[α]20/D +4°, c = 1.0242 in chloroform stab. with amylenes
mp
103-107 °C (lit.)
儲存溫度
2-8°C
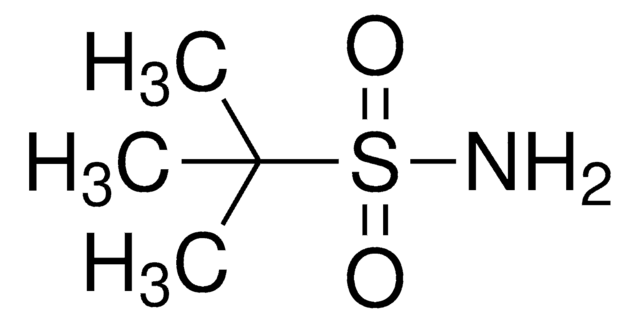
SMILES 字串
CC(C)(C)S(N)=O
InChI
1S/C4H11NOS/c1-4(2,3)7(5)6/h5H2,1-3H3/t7-/m1/s1
InChI 密鑰
CESUXLKAADQNTB-SSDOTTSWSA-N
一般說明
(R)-(+)-2-甲基-2-丙烷亚磺酰胺是一种手性助剂,用于醛的缩合。
應用
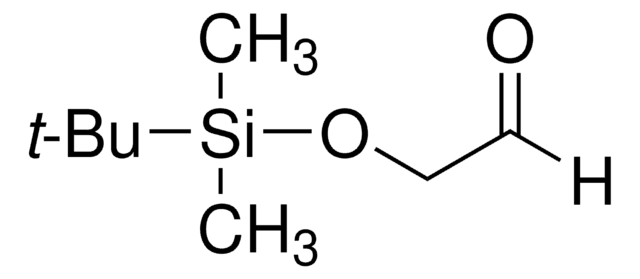
( R )-(+)-2-甲基-2-丙亚磺酰胺可用于通过铜介导与环己烷甲醛缩合制备 N -(1-环己基亚甲基)-2-甲基丙-2-亚磺酰胺。也可用于制备 (20E)-N-[t-丁基-(R)-亚磺酰基]-3β-( t -丁基二甲基硅氧烷)-孕甾-5-烯-20-亚胺,开发雄激素受体拮抗剂的中间体。
可通过与醛酮缩合易转化为 P,N -亚磺酰亚胺配体,后者可进行铱催化的烯烃不对称氢化反应。
手性氮杂环丁烷合成中 ß-氯亚磺酰胺的制备。也用于制备有机催化剂,用于亚胺的对映选择性还原。
用于合成手性胺的有用试剂。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
20-Aminosteroids as a novel class of selective and complete androgen receptor antagonists and inhibitors of prostate cancer cell growth.
Fousteris MA, et al.
Bioorganic & Medicinal Chemistry, 18(19), 6960-6969 (2010)
Asymmetric synthesis of a, a-difluoro-?-amino acid derivatives from enantiomerically pure N-tert-butylsulfinimines.
Staas, DD, et al.
The Journal of Organic Chemistry, 67(23), 8276-8279 (2002)
Dong Pei et al.
Organic letters, 8(25), 5913-5915 (2006-12-01)
Easily accessible chiral sulfinamide 2 has been developed as the first highly efficient and enantioselective organocatalyst relying solely on a chiral sulfur center for stereochemical induction. In the presence of 20 mol % of 2, a broad range of N-aryl
Bram Denolf et al.
Organic letters, 8(14), 3129-3132 (2006-06-30)
[reaction: see text] Reaction of chiral alpha-chloro tert-butanesulfinyl aldimines with Grignard reagents efficiently afforded beta-chloro N-sulfinamides in high diastereomeric excess. The latter compounds were cyclized toward the corresponding chiral aziridines in a high-yielding one-pot reaction or after separate treatment with
Organic Letters, 69, 1800-1800 (2004)
商品
Ellman's sulfinamide is available in both enantiomeric and racemic forms for your research. This versatile and useful auxiliary has found extensive use both in academics and industry.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持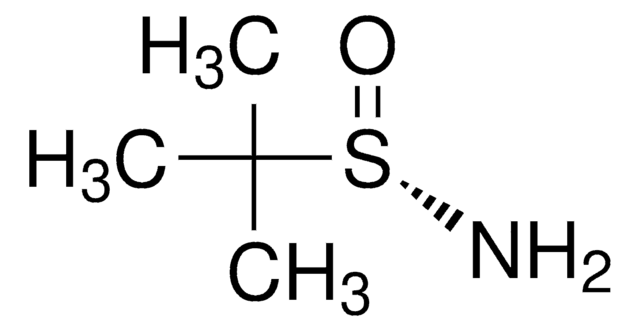



![(R)-N-[(1R,2R)-2-(3-(3,5-双(三氟甲基)苯基)脲基)环己基]-叔丁基亚磺酰胺 96%](/deepweb/assets/sigmaaldrich/product/structures/389/070/18847164-c6a7-4b4e-abcb-2dbc22493a2d/640/18847164-c6a7-4b4e-abcb-2dbc22493a2d.png)