About This Item
推荐产品
品質等級
化驗
95%
形狀
powder
反應適用性
reaction type: C-C Bond Formation
mp
75-79 °C
儲存溫度
2-8°C
SMILES 字串
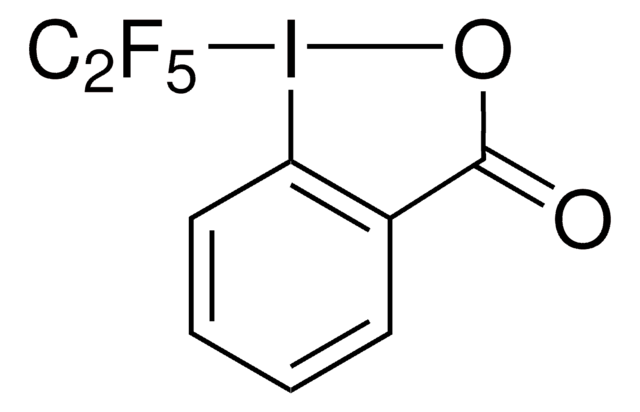
CC1(C)O[I](c2ccccc12)C(F)(F)F
InChI
1S/C10H10F3IO/c1-9(2)7-5-3-4-6-8(7)14(15-9)10(11,12)13/h3-6H,1-2H3
InChI 密鑰
HVAPLSNCVYXFDQ-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
多种化合物的三氟甲基化包括:
- 仲和伯芳基和烷基膦
- 酚类
- 经过SPPS和亲电子S-三氟甲基化的含有半胱氨酸的肽
- 芳烃和N-杂环
- Arozoles的亲电N-三氟甲基化
訊號詞
Warning
危險分類
Eye Irrit. 2 - Flam. Sol. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
4.1B - Flammable solid hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
商品
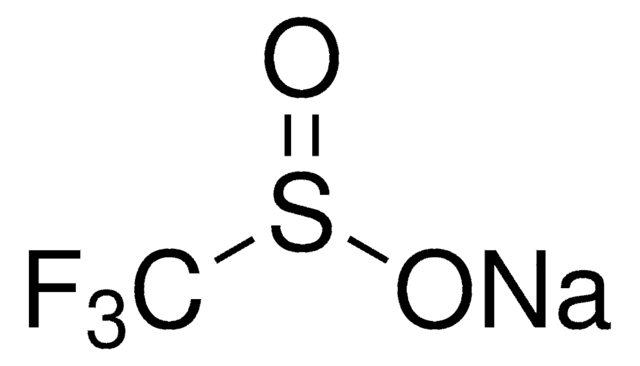
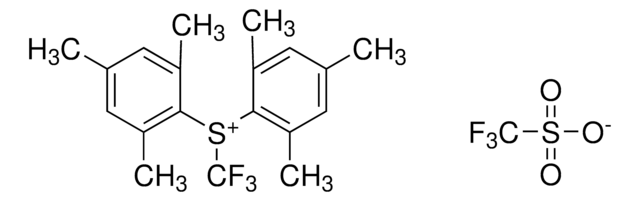
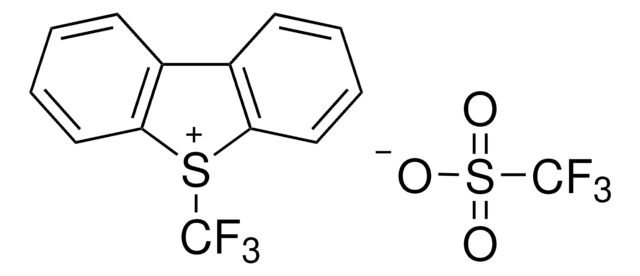
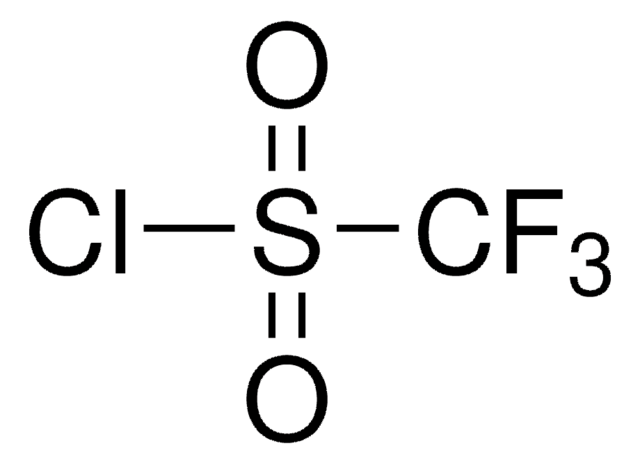
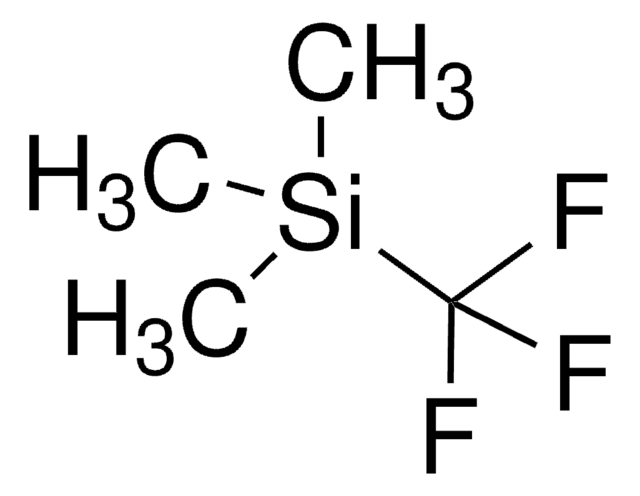
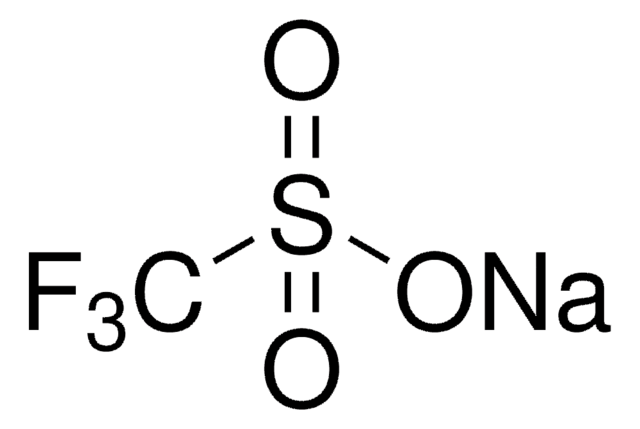
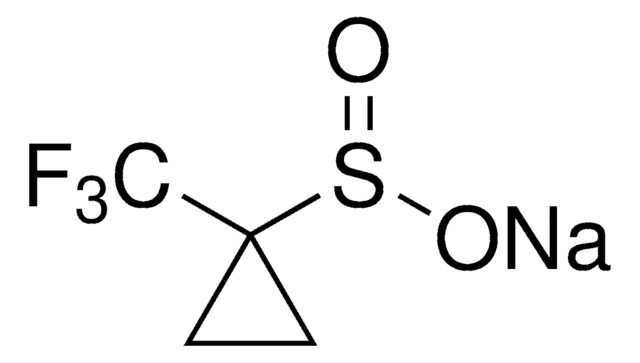
The fluoroalkylation toolbox now includes Togni reagents, hypervalent iodine perfluoroalkylation reagents, fluoroalkyl bromides, silanes, carboxylates, and sulfonyl fluorides for late stage fluoroalkylation.
目前的氟烷基化工具包括Togni试剂、高价碘全氟烷基化试剂、氟烷基溴化物、硅烷类和羧酸盐类以及用于后期氟烷基化的磺酰氟类。
The fluoroalkylation toolbox now includes Togni reagents, hypervalent iodine perfluoroalkylation reagents, fluoroalkyl bromides, silanes, carboxylates, and sulfonyl fluorides for late stage fluoroalkylation.
The fluoroalkylation toolbox now includes Togni reagents, hypervalent iodine perfluoroalkylation reagents, fluoroalkyl bromides, silanes, carboxylates, and sulfonyl fluorides for late stage fluoroalkylation.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门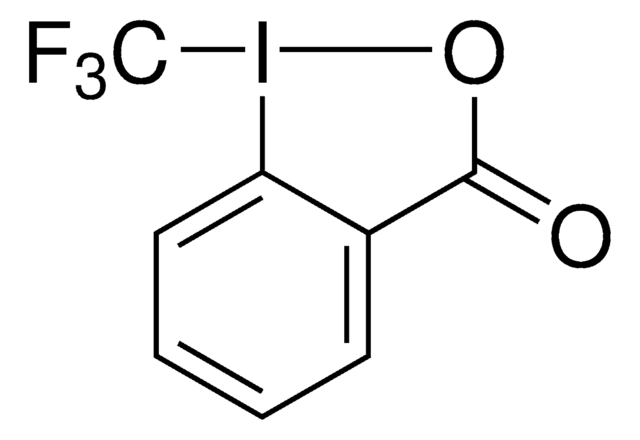
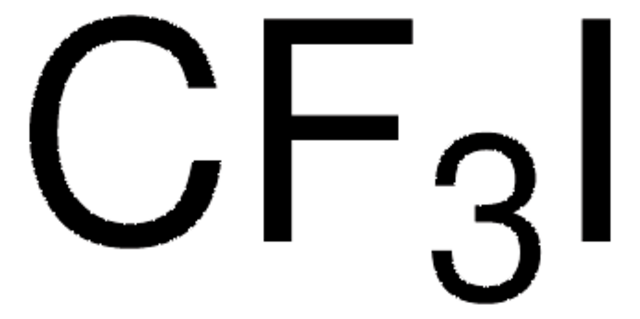


![1-[(Triisopropylsilyl)ethynyl]-1,2-benziodoxol-3(1H)-one ≥98.0% (AT)](/deepweb/assets/sigmaaldrich/product/structures/306/080/5d7d692f-a7c1-44ce-a2f9-ee6cffe8f42d/640/5d7d692f-a7c1-44ce-a2f9-ee6cffe8f42d.png)