所有图片(3)
About This Item
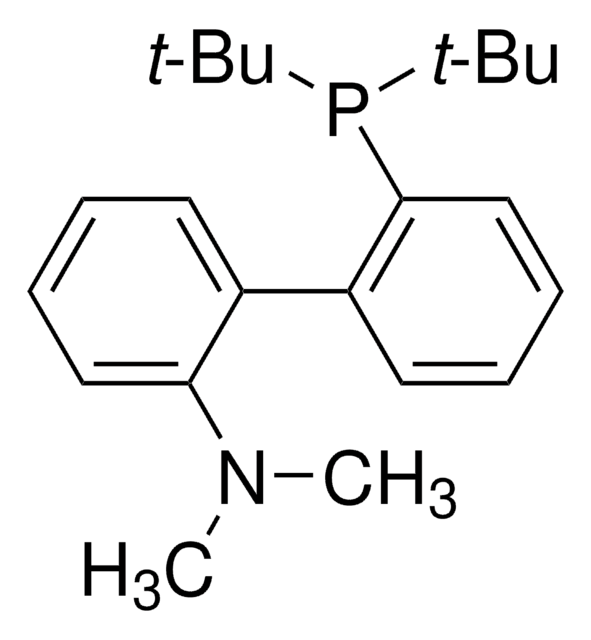
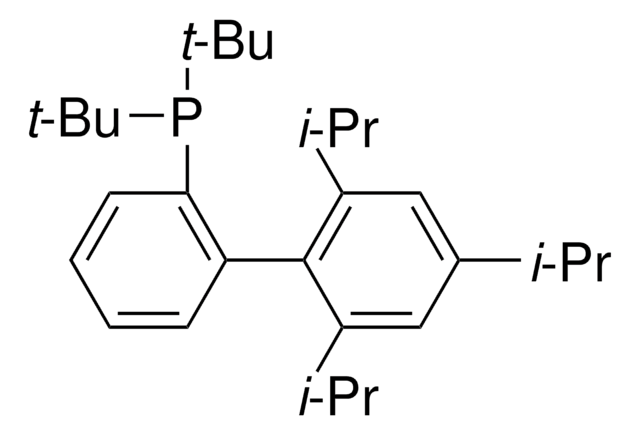
线性分子式:
C6H5C6H4P[C(CH3)3]2
CAS号:
分子量:
298.40
Beilstein:
8322131
MDL號碼:
分類程式碼代碼:
12352002
PubChem物質ID:
NACRES:
NA.22
推荐产品
品質等級
化驗
97%
反應適用性
reaction type: Cross Couplings
reagent type: ligand
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reagent type: ligand
reaction type: C-X Bond Formation
reagent type: ligand
reaction type: Heck Reaction
reagent type: ligand
reaction type: Suzuki-Miyaura Coupling
mp
86-88 °C (lit.)
官能基
phosphine
SMILES 字串
CC(C)(C)P(c1ccccc1-c2ccccc2)C(C)(C)C
InChI
1S/C20H27P/c1-19(2,3)21(20(4,5)6)18-15-11-10-14-17(18)16-12-8-7-9-13-16/h7-15H,1-6H3
InChI 密鑰
CNXMDTWQWLGCPE-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
JohnPhos 是Buchwald空间庞大的二芳基膦配体。它是一种反应性二烷基联芳基膦配体,可催化碳-氮键形成反应。带有JohnPhos作为配体的金催化剂的配位化学已通过NMR光谱进行了研究。
了解更多有关Buchwald膦配体的信息
了解更多有关Buchwald膦配体的信息
應用
JohnPhos是一种庞大的膦配体,在以下研究中被用作催化剂:
- 未活化的内部炔烃的氢苯氧基化。
- 微波介导的苄基溴的Suzuki-Miyaura交叉偶联。
- 新型咪唑并[1,2-a]吡啶药物的合成,其对疱疹病毒具有强效活性。
- 将乙烯基溴与肼进行Barluenga′s偶联。
- Pd催化的α,α--二取代-3-噻吩甲醇的2,3-二芳基化(通过裂解CH和CC键)。
作为配体,其用于芳基卤化物和芳基三氟甲磺酸酯的胺化。
可催化:
可催化:
- 二烷氧基苯甲酸与二芳基二硫化物或二芳基二硒醚的脱羧交叉偶联
- 通过N-烯丙基-N-芳基脲的分子内加氢胺化,立体选择性制备咪唑烷酮
- 烯烃与芳基氯化物的区域选择性芳基化
- 用于合成多不饱和大环内酯的交叉偶联反应
- 区域选择性 O-烷基化反应
- Sonogashira型交叉偶联
钯催化的Stille交叉偶联反应所用的大位阻联芳基膦配体。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
Artamkina, Galina A.; et al.
Synlett, 2, 235-238 (2006)
Steven W McDaniel et al.
Tetrahedron letters, 52(43), 5656-5658 (2011-10-04)
A procedure for benzylic Suzuki-Miyaura cross-coupling under microwave conditions has been developed. These conditions allowed for heterocyclic compounds to be coupled. Optimum conditions found were Pd(OAc)(2), JohnPhos as the catalyst and ligand, potassium carbonate as base, and DMF as the
Alexander Zhdanko et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 18(46), 14732-14744 (2012-09-29)
Coordination chemistry of gold catalysts bearing eight different ligands [L=PPh(3), JohnPhos (L2), Xphos (L3), DTBP, IMes, IPr, dppf, S-tolBINAP (L8)] has been studied by NMR spectroscopy in solution at room temperature. Cationic or neutral mononuclear complexes LAuX (L=L2, L3, IMes
Marcia E Richard et al.
Beilstein journal of organic chemistry, 9, 2002-2008 (2013-11-10)
A range of arylgold compounds have been synthesized and investigated as single-component catalysts for the hydrophenoxylation of unactivated internal alkynes. Both carbene and phosphine-ligated compounds were screened as part of this work, and the most efficient catalysts contained either JohnPhos
Masaya Nakano et al.
The Journal of organic chemistry, 71(21), 8309-8311 (2006-10-10)
Alpha,alpha-disubstituted 3-thiophenemethanols undergo selective diarylation accompanied by cleavage of the C-H and C-C bonds of the 2- and 3-positions, respectively, upon treatment with aryl bromides in the presence of a palladium catalyst to give the corresponding 2,3-diarylthiophenes in good yields.
商品
Global Trade Item Number
| 货号 | GTIN |
|---|---|
| 638439-25G | 4061833399354 |
| 638439-100G | 4061833301548 |
| 638439-5G | 4061833357019 |
| 638439-1G | 4061832726700 |
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持



![氯[2-(二叔丁基磷)二苯基]金 98%](/deepweb/assets/sigmaaldrich/product/structures/192/300/6b6ed2fd-ac0b-4370-80e3-807dad65a825/640/6b6ed2fd-ac0b-4370-80e3-807dad65a825.png)