所有图片(1)
About This Item
经验公式(希尔记法):
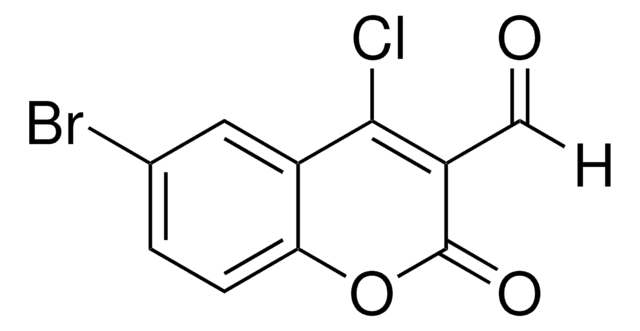
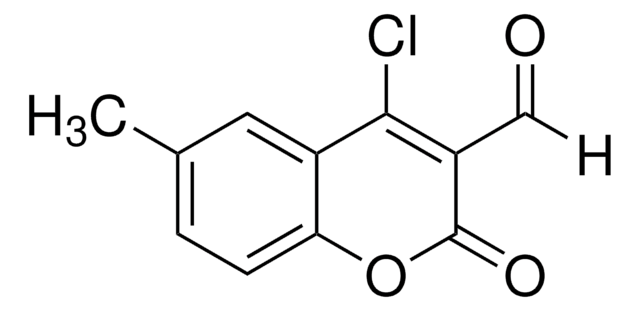
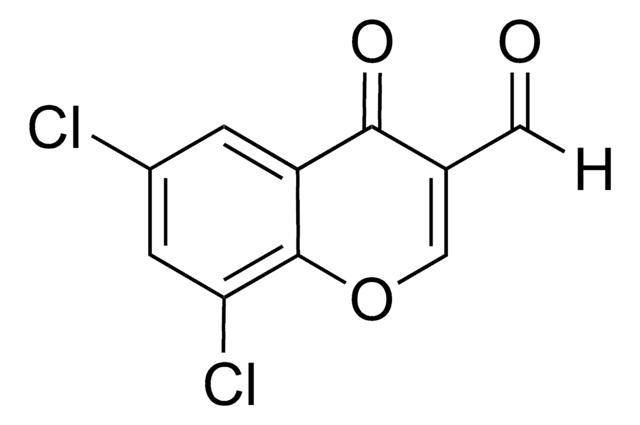
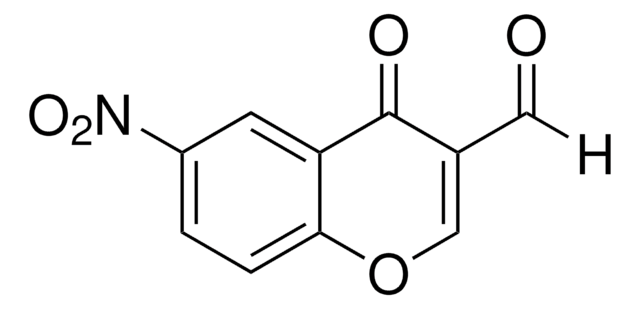
C10H5ClO3
CAS号:
分子量:
208.60
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
一般說明
4-Chloro-3-formylcoumarin, also known as 4-chloro-2-oxo-2H-chromene-3-carbaldehyde, is a coumarin derivative.
應用
4-Chloro-3-formylcoumarin may be used as a reactant in the preparation of:
- 2-aryl[1]benzopyrano[4,3-c]pyrazol-4(2H)-ones
- biaryl lactones (benzo[c]chromen-6-ones)
- N-monosubstituted 4-amino-3-formylcoumarins
- chromeno[4,3-b]quinolin-6-one
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Synthesis of 6H-benzo [c] chromen-6-ones by cyclocondensation of 1,3-dicarbonyl compounds with 4-chloro-3-formylcoumarin.
Iaroshenko, VO, et al.
Tetrahedron Letters, 52(45), 5910-5912 (2011)
An efficient ultrasound promoted catalyst-free protocol for the synthesis of chromeno [4,3-b] quinolin-6-ones.
Prasad JV, et al.
Chemical Science, 123(5), Prasad JV-Prasad JV (2011)
Reactions of 4-Chloro-3-formylcoumarin with Arylhydrazines.
Strakova I, et al.
Chemistry of Heterocyclic Compounds, 39(12), 1608-1616 (2003)
Reactions of 4-chloro-3-formyl-coumarin with primary amines.
Strakova I, et al.
Chemistry of Heterocyclic Compounds, 42(5), 574-582 (2006)
One-Pot Synthesis of Biaryl Lactones by Sonogashira Cross-Coupling Reactions of 4-Chloro-3-formylcoumarin and Subsequent Domino [5+1] Cyclization/Deacetylation Reactions with 1, 3-Dicarbonyl Compounds.
Iaroshenko VO, et al.
Advanced Synthesis & Catalysis, 354(5), 803-806 (2012)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门