所有图片(2)
About This Item
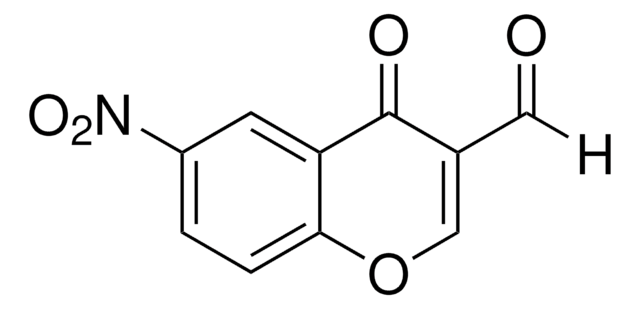
经验公式(希尔记法):
C10H6O3
CAS号:
分子量:
174.15
EC號碼:
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
97%
形狀
solid
mp
151-153 °C (lit.)
官能基
aldehyde
ketone
SMILES 字串
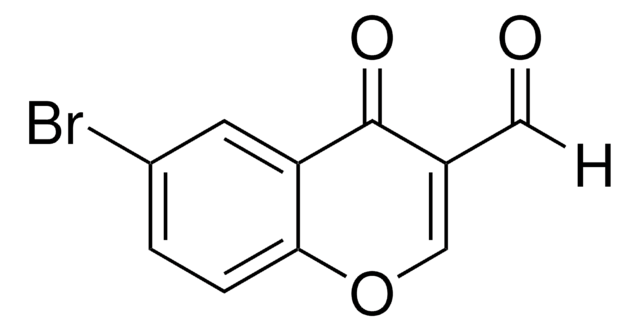
O=CC1=COc2ccccc2C1=O
InChI
1S/C10H6O3/c11-5-7-6-13-9-4-2-1-3-8(9)10(7)12/h1-6H
InChI 密鑰
FSMYWBQIMDSGQP-UHFFFAOYSA-N
基因資訊
human ... PTPN1(5770)
相关类别
一般說明
质子化 3-甲酰色原酮 (3-FC) 的电喷雾离子化质谱 (ESI-MS) 显示丢失 H 2 是生成烯酮阳离子的主要裂解途径,与水反应生成质子化羧酸。研究了 3-FC 对亚硝基二乙胺 (NDEA) 介导的早期肝细胞癌变的 体内 有益作用。3-FC 及其衍生物的合成与表征已有报告。
應用
3-甲酰色原酮可用于以下研究:
- ( E )-3-(2-芳基羰基-3-(芳基氨基)烯丙基)-4 H -色烯-4-酮与 ( E )-3-(二甲氨基)-1-芳基脯氨酸-2-烯-1-酮和苯胺在无催化剂条件下的三组分多米诺反应制备文库。
- 新型铬酮类化合物的合成。
- 3-(2-羟基苯甲酰基)喹啉和 7 H -色胺诺 [3,2- c ] 喹啉-7-酮的合成。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
Koichi Takao et al.
Bioorganic chemistry, 83, 432-437 (2018-11-15)
A series of eighteen pyrano[4,3-b][1]benzopyranone derivatives (1a-9b) were synthesized, and structure-activity relationships of their monoamine oxidase (MAO) A and B, acetylcholinesterase (AChE), and butyrylcholinesterase (BChE) inhibitory activities were evaluated. Most of the synthesized compounds exhibited weak inhibitory activity toward MAO-A
Pedatsur Neta et al.
Rapid communications in mass spectrometry : RCM, 28(17), 1871-1882 (2014-08-05)
Electrospray ionization mass spectrometry (ESI-MS) of many protonated aldehydes shows loss of CO as a major fragmentation pathway. However, we find that certain aldehydes undergo loss of H2 followed by reaction with water in the collision cell. This complicates interpretation
Andrey S Plaskon et al.
The Journal of organic chemistry, 73(15), 6010-6013 (2008-07-03)
A facile and versatile procedure for the synthesis of 3-(2-hydroxybenzoyl)quinolines and 7H-chromeno[3,2-c]quinolin-7-ones was elaborated on the basis of TMSCl-mediated recyclization of 3-formylchromone with various anilines. Limitations and scope of this methodology were established, and a possible mechanism for the heterocyclizations
Pitchaimani Prasanna et al.
Beilstein journal of organic chemistry, 10, 459-465 (2014-03-13)
The three-component domino reactions of (E)-3-(dimethylamino)-1-arylprop-2-en-1-ones, 3-formylchromone and anilines under catalyst-free conditions afforded a library of novel (E)-3-(2-arylcarbonyl-3-(arylamino)allyl)-4H-chromen-4-ones in good to excellent yields and in a diastereoselective transformation. This transformation generates one C-C and one C-N bond and presumably proceeds
Zeba N Siddiqui et al.
Journal of enzyme inhibition and medicinal chemistry, 27(1), 84-91 (2011-05-27)
A facile and ecofriendly synthesis of new chromonyl chalcones 3a-b from 3-formylchromone 1 and active methyl compounds 2a-b is reported under thermal solvent-free heating condition in good yields. The chromonyl chalcones 3a-b were used as intermediates under green condition for
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门