推荐产品
品質等級
化驗
95%
折射率
n20/D 1.495 (lit.)
bp
195 °C (lit.)
密度
1.013 g/mL at 25 °C (lit.)
官能基
ether
SMILES 字串
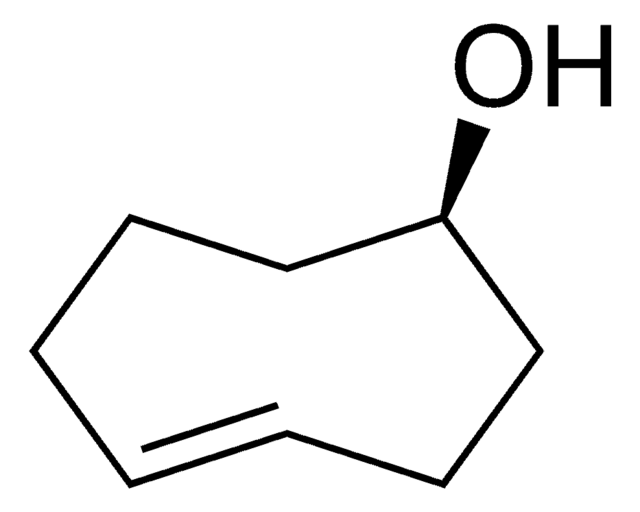
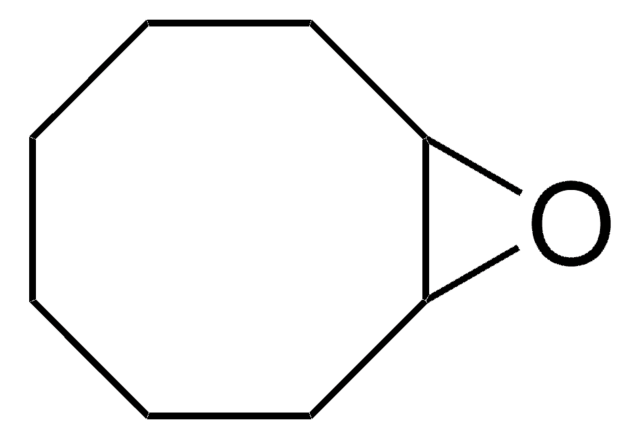
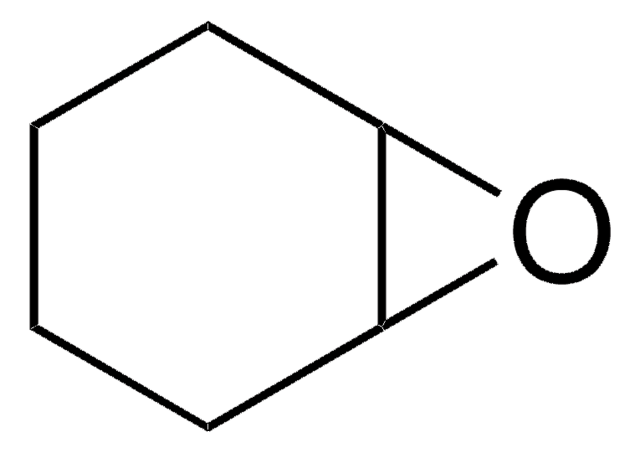
C1CC2OC2CCC=C1
InChI
1S/C8H12O/c1-2-4-6-8-7(9-8)5-3-1/h1-2,7-8H,3-6H2/b2-1-
InChI 密鑰
YWFPXWMSGJXUFS-UPHRSURJSA-N
一般說明
9-Oxabicyclo[6.1.0]non-4-ene is an epoxide. It undergoes halofluorination reactions with N-halosuccinimides and triethylamine tris-hydrofluoride or Olah′s reagent.
應用
9-Oxabicyclo[6.1.0]non-4-ene may be used in the following studies:
- novel bicyclo[4.2.1] and bicyclo[3.3.1] ethers
- trans, trans-2,6-dibromo-9-oxabicyclo[3.3.1]nonane and trans, trans-2,5-dibromo-9-oxabicyclo[4.2.1]nonane
- (Z)-2-(cyclooct-4-enyloxy)acetic acid.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
158.0 °F - closed cup
閃點(°C)
70 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
Karl J Bonney et al.
The Journal of organic chemistry, 76(1), 97-104 (2010-12-01)
9-Oxabicyclo[6.1.0]non-4-ene (1) undergoes intramolecular bromonium ion-assisted epoxide ring-opening using N-bromosuccinimide via a presumed oxonium ion that is subject to stereospecific, nonregioselective capture with added external nucleophiles producing novel bicyclo[4.2.1] and bicyclo[3.3.1] ethers. Carboxylic acids (as catalyzed by tetramethylguanidine), alcohols, water
Stereoselective synthesis of cyclic ethers via bromine assistedepoxide ring expansion.
Davies SG, et al.
Tetrahedron Letters, 26(11), 1461-1464 (1985)
Transannular oxygen participation in halofluorination reactions of 9-oxabicyclo [6.1. 0] non-4-ene [1].
Haufe G, et al.
Journal of Fluorine Chemistry, 46(1), 83-95 (1990)
High-yielding, two-step 18F labeling strategy for 18F-PARP1 inhibitors.
Edmund J Keliher et al.
ChemMedChem, 6(3), 424-427 (2011-03-02)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门