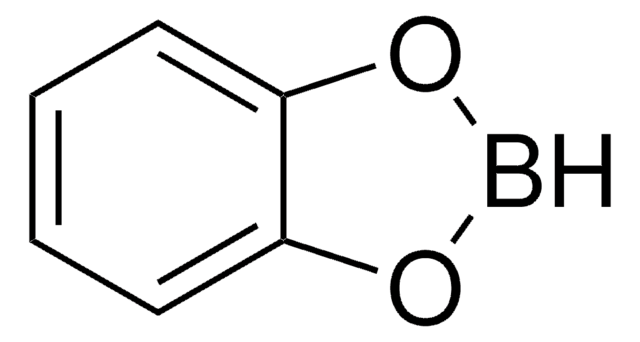
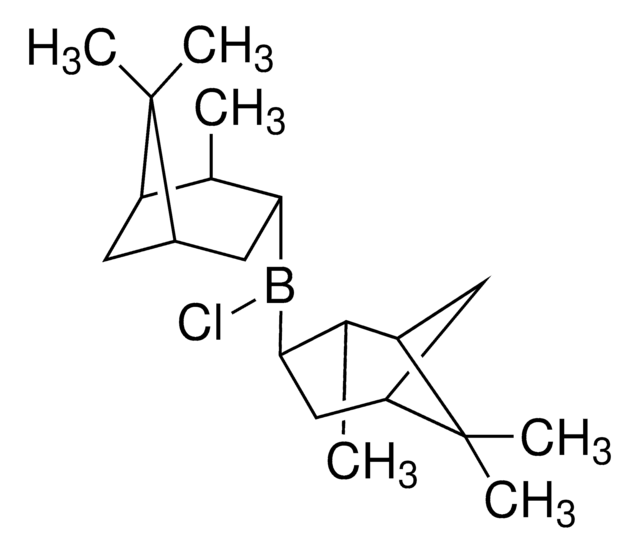
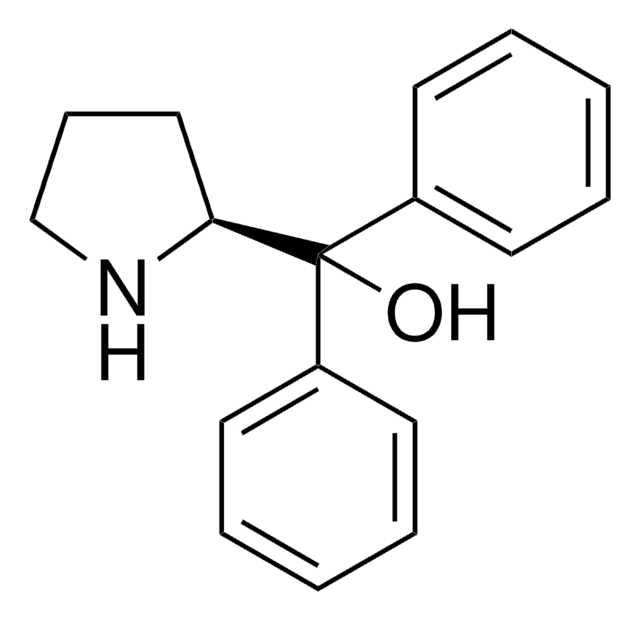
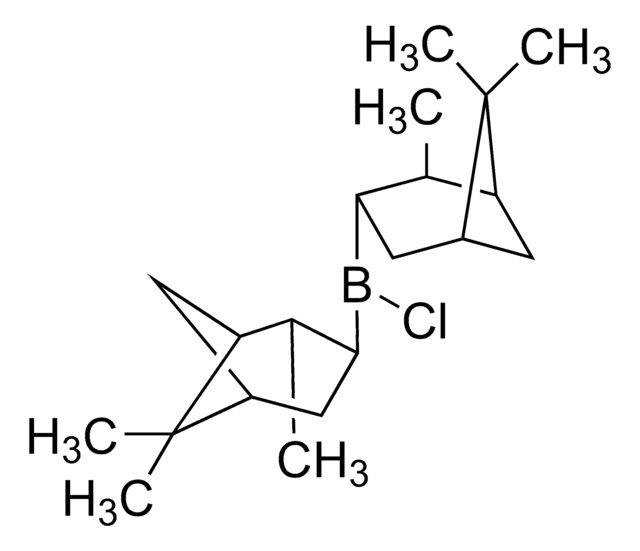
推荐产品
品質等級
濃度
1 M in toluene
bp
111 °C
密度
0.95 g/mL at 25 °C
官能基
phenyl
儲存溫度
room temp
SMILES 字串
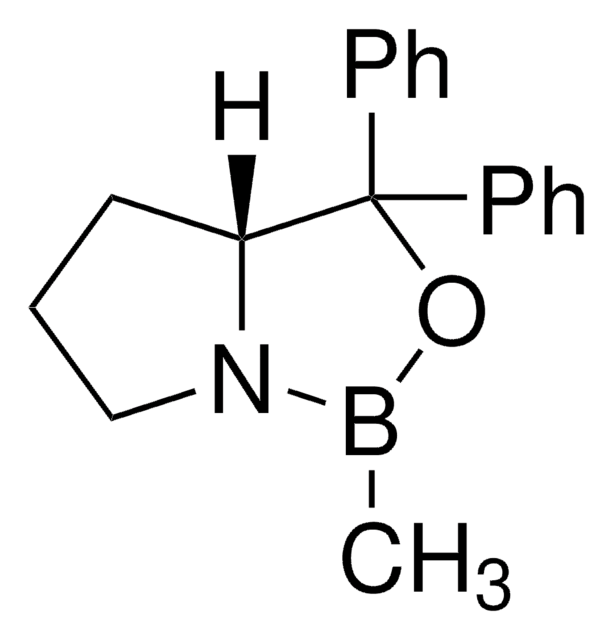
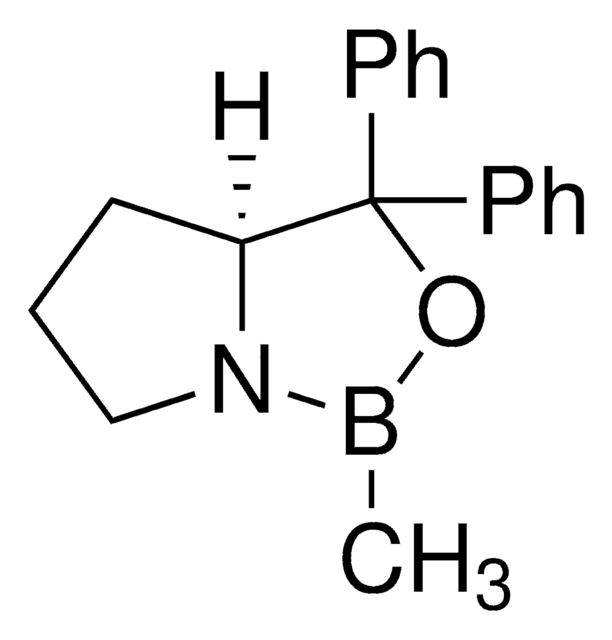
[H][C@]12CCCN1B(C)OC2(c3ccccc3)c4ccccc4
InChI
1S/C18H20BNO/c1-19-20-14-8-13-17(20)18(21-19,15-9-4-2-5-10-15)16-11-6-3-7-12-16/h2-7,9-12,17H,8,13-14H2,1H3/t17-/m1/s1
InChI 密鑰
VMKAFJQFKBASMU-QGZVFWFLSA-N
正在寻找类似产品? 访问 产品对比指南
應用
用于脱对称还原反应,生成(S)-4-羟基环己烯酮。
它也可以用于制备:
- (-)-地黄皂苷 B
- (1R)-2-叠氮基-1-(2,2-二甲基-4H-1,3-苯并二恶英-6-基)乙醇
- (S)-α-氘代苄醇
- (3S,4R,5S)-1-(三甲基甲硅烷基)-4,5-环氧己基-1-yn-3-醇
外觀
訊號詞
Danger
危險分類
Aquatic Chronic 3 - Asp. Tox. 1 - Eye Dam. 1 - Flam. Liq. 2 - Repr. 2 - Skin Irrit. 2 - STOT RE 2 - STOT SE 3
標靶器官
Central nervous system
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
39.2 °F - closed cup
閃點(°C)
4 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
商品
we are pleased to offer both enatiomers of 2-methyl-CBS-oxazaborolidine as a dry reagent, in addition to our current offerings as a 1M solution in toluene.
We are pleased to offer o-tolyl-CBS-oxazaborolidine as a 0.5 M solution in toluene for your research needs. When protonated with trifluoromethanesulfonimide, these chiral oxazaborolidines generate chiral Lewis acids, which have demonstrated great utility in the enantioselective Diels–Alder reaction.
We are pleased to offer o-tolyl-CBS-oxazaborolidine as a 0.5 M solution in toluene for your research needs. When protonated with trifluoromethanesulfonimide, these chiral oxazaborolidines generate chiral Lewis acids, which have demonstrated great utility in the enantioselective Diels–Alder reaction.
we are pleased to offer both enatiomers of 2-methyl-CBS-oxazaborolidine as a dry reagent, in addition to our current offerings as a 1M solution in toluene.
相关内容
Our company is pleased to offer both enantiomers of 2-methyl-CBS-oxazaborolidine as a dry reagent, in addition to our current offerings as a 1 M solution in toluene.
Our company is pleased to offer both enantiomers of 2-methyl-CBS-oxazaborolidine as a dry reagent, in addition to our current offerings as a 1 M solution in toluene.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门