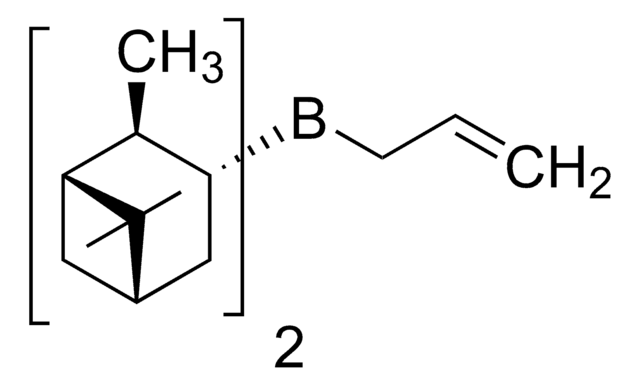
推荐产品
品質等級
化驗
97%
光學活性
[α]21/D -22°, c = 12 in THF
bp
>55 °C (lit.)
密度
0.947 g/mL at 25 °C (lit.)
SMILES 字串
C[C@H]1[C@@H](CC2CC1C2(C)C)B3C4CCCC3CCC4
InChI
1S/C18H31B/c1-12-16-10-13(18(16,2)3)11-17(12)19-14-6-4-7-15(19)9-5-8-14/h12-17H,4-11H2,1-3H3/t12-,13+,14-,15+,16-,17-/m1/s1
InChI 密鑰
VCDGSBJCRYTLNU-PHPOFCCKSA-N
一般說明
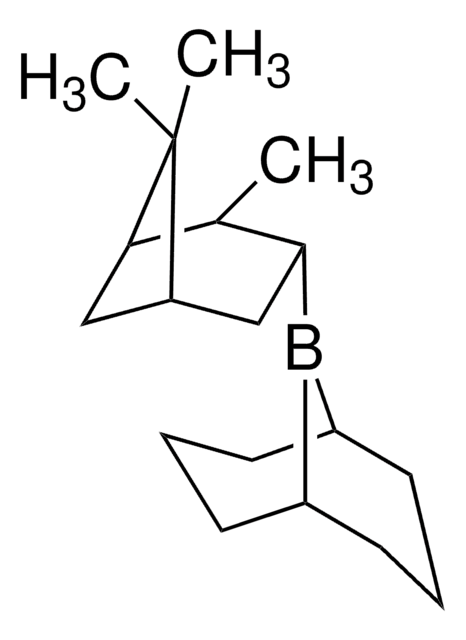
R-Alpine-Borane® is a chiral reducing agent, synthesized from (+)-α-pinene via hydroboration.
應用
用于多种前手性酮的不对称还原反应试剂。
R-Alpine-Borane® may be used in the preparation of (22R)-hydroxy-23-acetylenic steroids with high stereoselectivity.
法律資訊
Alpine-Borane is a registered trademark of Merck KGaA, Darmstadt, Germany
訊號詞
Danger
危險聲明
危險分類
Pyr. Liq. 1
儲存類別代碼
4.2 - Pyrophoric and self-heating hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Ramachandran, P.V. et al.
Tetrahedron Asymmetry, 4, 2399-2399 (1993)
Stereocontrolled synthesis of 22-hydroxy-23-acetylenic steroids, key intermediates in steroid side chain construction. Observation of a directive effect by an a-chiral site during asymmetric reduction with-B-3-pinanyl-9-BBN (Alpine-Borane).
Midland MM and Kwon YC.
Tetrahedron Letters, 25(52), 5981-5984 (1984)
Diisopinocampheylchloroborane, a remarkably efficient chiral reducing agent for aromatic prochiral ketones.
Chandrasekharan J, et al.
The Journal of Organic Chemistry, 50(25), 5446-5448 (1985)
Matteson DS
Stereodirected Synthesis with Organoboranes, 346-347 (2012)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门