About This Item
推荐产品
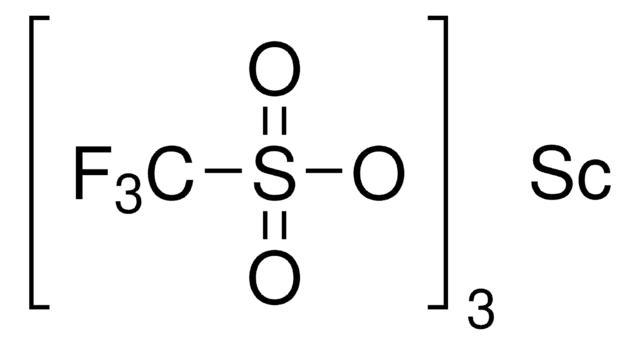
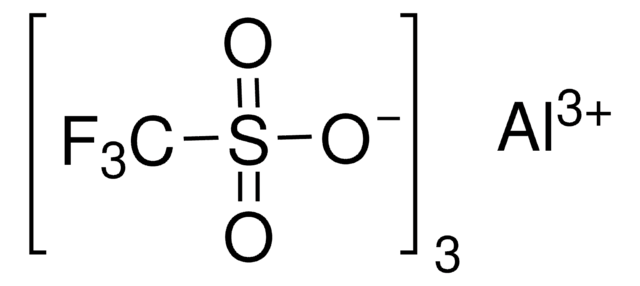
成份
Degree of hydration, 1-2
Yb, 25-28% (approx.)
反應適用性
core: ytterbium
reagent type: catalyst
SMILES 字串
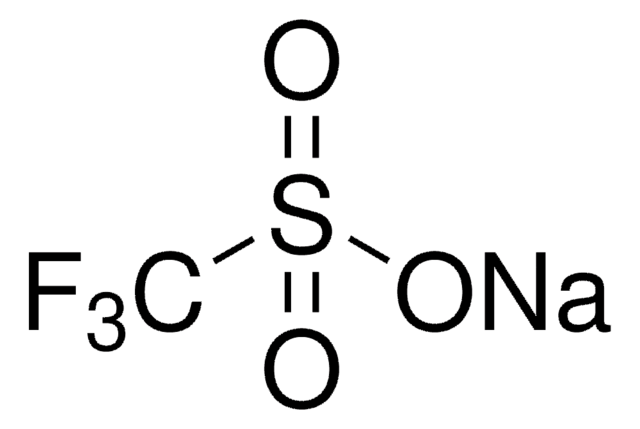
[H]O[H].FC(F)(F)S(=O)(=O)O[Yb](OS(=O)(=O)C(F)(F)F)OS(=O)(=O)C(F)(F)F
InChI
1S/3CHF3O3S.H2O.Yb/c3*2-1(3,4)8(5,6)7;;/h3*(H,5,6,7);1H2;/q;;;;+3/p-3
InChI 密鑰
BUJKNFNMGRYZBV-UHFFFAOYSA-K
正在寻找类似产品? 访问 产品对比指南
一般說明
應用
- 通过Biginelli环缩合反应,合成二氢嘧啶(DHPM)衍生物monastrol。
- 酮与醛的交叉醛醇反应。
- 合成β-酮烯醇醚。
- 在新型立体选择性分子内Diels-Alder反应中,合成deoxypenostatin A。
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
商品
The Friedel–Crafts acylation is the reaction of an arene with acyl chlorides or anhydrides using a strong Lewis acid catalyst. This reaction proceeds via electrophilic aromatic substitution to form monoacylated products.
The Friedel–Crafts acylation is the reaction of an arene with acyl chlorides or anhydrides using a strong Lewis acid catalyst. This reaction proceeds via electrophilic aromatic substitution to form monoacylated products.
Global Trade Item Number
| 货号 | GTIN |
|---|---|
| 405329-5G | 4061833322987 |
| 405329-25G | 4061833424803 |
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持