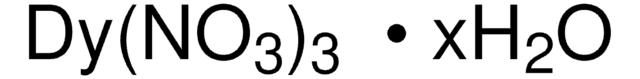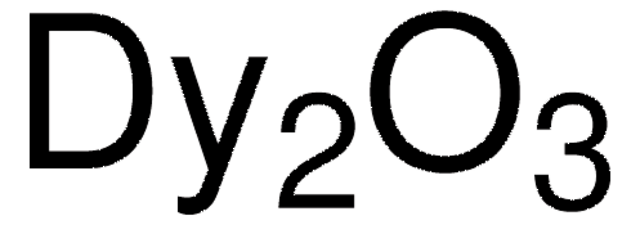About This Item
推荐产品
化驗
98%
反應適用性
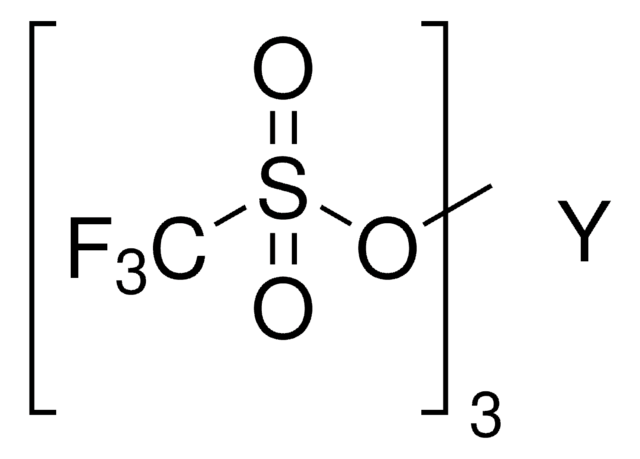
core: dysprosium
reagent type: catalyst
reaction type: Ring-Opening Polymerization
SMILES 字串
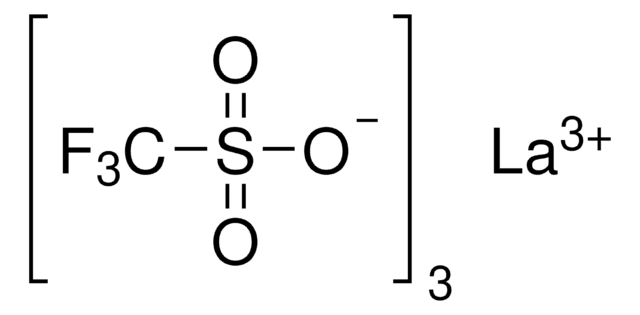
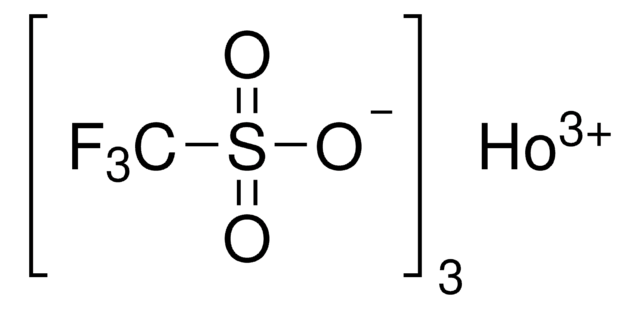
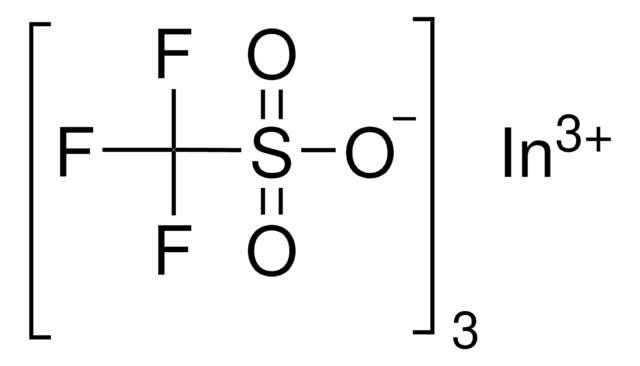
[Dy+3].[O-]S(=O)(=O)C(F)(F)F.[O-]S(=O)(=O)C(F)(F)F.[O-]S(=O)(=O)C(F)(F)F
InChI
1S/3CHF3O3S.Dy/c3*2-1(3,4)8(5,6)7;/h3*(H,5,6,7);/q;;;+3/p-3
InChI 密鑰
XSVCYDUEICANRJ-UHFFFAOYSA-K
一般說明
應用
- 烯醇硅醚与醛的醛醇缩合反应。
- 作为吲哚与亚胺的亲电取代反应的有效催化剂。
- 作为通过分子内氮杂-Piancatelli重排合成4-氨基环戊烯酮 和官能化氮杂螺环的催化剂。
- 作为研究双酚A二缩水甘油醚(DGEBA)固化的新型固化引发剂。
- Aza-Piancatelli重排
- Friedel-Crafts烷基化
- 开环聚合反应
- 微波辅助的Kabachnik-Fields缩合反应
- 环加成反应(路易斯酸催化剂)
- Fries 重排
- 对映选择性glyoxalate-ene反应
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
商品
The Fries rearrangement reaction is an organic name reaction which involves the conversion of phenolic esters into hydroxyaryl ketones on heating in the presence of a catalyst. Suitable catalysts for this reaction are Brønsted or Lewis acids such as HF, AlCl3, BF3, TiCl4, or SnCl4. The Fries rearrangement reaction is an ortho, para-selective reaction, and is used in the preparation of acyl phenols. This organic reaction has been named after German chemist Karl Theophil Fries.
The Fries rearrangement reaction is an organic name reaction which involves the conversion of phenolic esters into hydroxyaryl ketones on heating in the presence of a catalyst. Suitable catalysts for this reaction are Brønsted or Lewis acids such as HF, AlCl3, BF3, TiCl4, or SnCl4. The Fries rearrangement reaction is an ortho, para-selective reaction, and is used in the preparation of acyl phenols. This organic reaction has been named after German chemist Karl Theophil Fries.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门