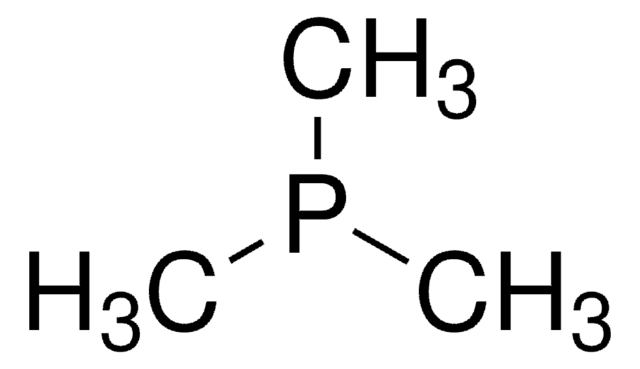
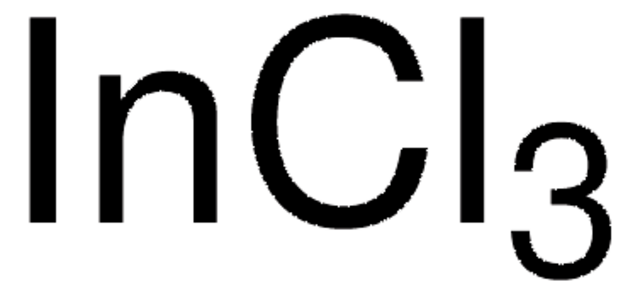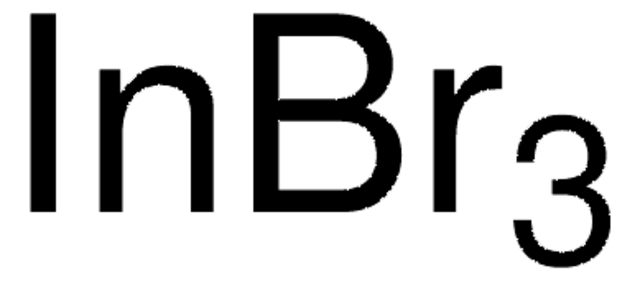推荐产品
品質等級
化驗
97%
形狀
liquid
反應適用性
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: ligand
折射率
n20/D 1.475 (lit.)
bp
80-90 °C/10 mmHg (lit.)
密度
0.903 g/mL at 25 °C (lit.)
官能基
phosphine
SMILES 字串
CCN(CC)P(N(CC)CC)N(CC)CC
InChI
1S/C12H30N3P/c1-7-13(8-2)16(14(9-3)10-4)15(11-5)12-6/h7-12H2,1-6H3
InChI 密鑰
FDIOSTIIZGWENY-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
三(二乙氨基)膦[(Et2N)3P]可作为试剂,合成以下物质:
也可用于通过富勒烯C60对某些环状 α-二酮进行脱氧。
- 1,1′-二烷基异靛蓝衍生物,通过脱氧反应与各种1-烷基异丁烯反应进行合成。
- 1-氨基甲基靛红,通过靛红与伯胺和仲胺反应合成。
也可用于通过富勒烯C60对某些环状 α-二酮进行脱氧。
訊號詞
Warning
危險分類
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
138.2 °F - closed cup
閃點(°C)
59 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
Khadga J Karki et al.
Scientific reports, 3, 2287-2287 (2013-07-28)
Multiple exciton generation (MEG) is a process in which more than one exciton is generated upon the absorption of a high energy photon, typically higher than two times the band gap, in semiconductor nanocrystals. It can be observed experimentally using
Facile synthesis of 1, 1′-dialkylisoindigos through deoxygenation reaction of isatins and tris (diethylamino) phosphine
Bogdanov AV, et al.
Synthesis, 2010(19), 3268- 3270 (2010)
Novel 1-Aminomethylisatins: Peculiarities of the Synthesis and the Reaction with Tris (diethylamino) phosphine
Bogdanov AV, et al.
Journal of Heterocyclic Chemistry, 51(4), 1027- 1030 (2014)
Irina P Romanova et al.
The Journal of organic chemistry, 76(8), 2548-2557 (2011-03-12)
The reactions of such cyclic α-diketones as acenaphthenequinone, aceanthrenequinone, and N-alkylisatins, with hexaethyltriaminophosphine in the presence of the fullerene C(60), lead to the formation of methanofullerene derivatives under mild conditions. This process proceeds via deoxygenation of the dicarbonyl compound by
K Yamana et al.
Nucleic acids symposium series, (21)(21), 31-32 (1989-01-01)
Utilities of deoxyribonucleoside 3'-O-phosphorbisdiethylamidites in the synthesis of oligodeoxyribonucleotides and their analogues are described.
Global Trade Item Number
| 货号 | GTIN |
|---|---|
| 253189-25G | 4061825982151 |
| 253189-5G | 4061838047380 |
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持