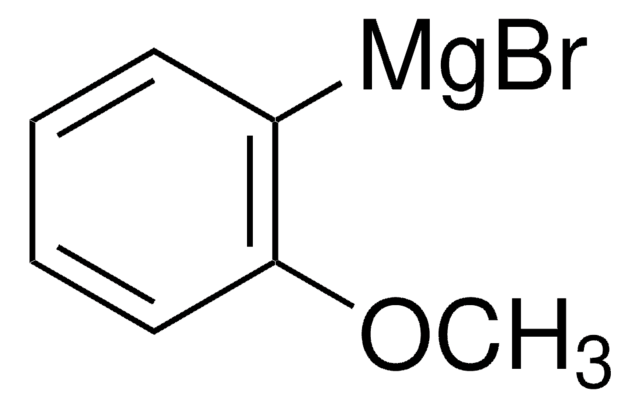
推荐产品
反應適用性
reaction type: Grignard Reaction
濃度
1.0 M in THF
密度
1.005 g/mL at 25 °C
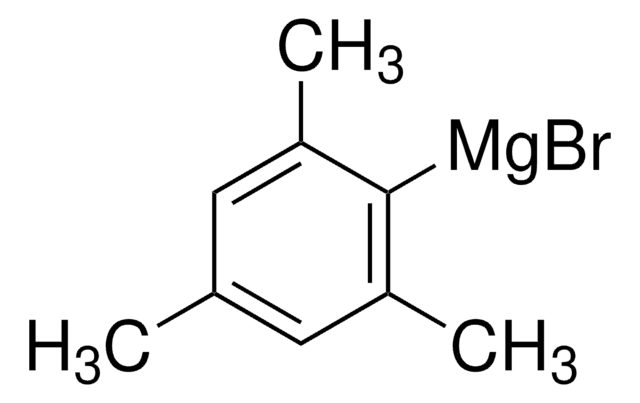
SMILES 字串
Cc1cc(C)c([Mg]Br)c(C)c1
InChI
1S/C9H11.BrH.Mg/c1-7-4-8(2)6-9(3)5-7;;/h4-5H,1-3H3;1H;/q;;+1/p-1
InChI 密鑰
YXVSITSUDRGILL-UHFFFAOYSA-M
正在寻找类似产品? 访问 产品对比指南
應用
2-三甲基溴化镁是一种Grignard试剂,可通过动力学阻断反应性分子位点,通常用于合成稳定的芳香族化合物,如二苯并噻吩、 二茚并蒽、 萜烯 和二甲基苯基[2,1-a]芴。它还可用于各种芳族交叉偶联反应。
訊號詞
Danger
危險分類
Carc. 2 - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B - STOT SE 3
標靶器官
Central nervous system, Respiratory system
安全危害
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
1.4 °F - closed cup
閃點(°C)
-17.0 °C - closed cup
其他客户在看
Iron-catalyzed selective biaryl coupling: Remarkable suppression of homocoupling by the fluoride anion.
Hatakeyama T and Nakamura M
Journal of the American Chemical Society, 129(32), 9844-9845 (2007)
Dibenzoheptazethrene isomers with different biradical characters: an exercise of Clar?s aromatic sextet rule in singlet biradicaloids.
Sun, Zhe et al.
Journal of the American Chemical Society, 135(48), 18229-18236 (2013)
Indeno [2, 1-a] fluorene: An Air-Stable ortho-Quinodimethane Derivative.
Shimizu A and Tobe Y
Angewandte Chemie (International Edition in English), 123(30), 7038-7042 (2011)
Diindeno-fusion of an anthracene as a design strategy for stable organic biradicals.
Rudebusch, Gabriel E et al.
Nature Chemistry, 8(8), 753-753 (2016)
Highly selective biaryl cross-coupling reactions between aryl halides and aryl Grignard reagents: a new catalyst combination of N-heterocyclic carbenes and iron, cobalt, and nickel fluorides.
Hatakeyama T, et al.
Journal of the American Chemical Society, 131(33), 11949-11963 (2009)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门