推荐产品
品質等級
化驗
95%
形狀
liquid
折射率
n20/D 1.418 (lit.)
bp
194-196 °C (lit.)
密度
1.035 g/mL at 25 °C (lit.)
官能基
amine
ester
isonitrile
儲存溫度
2-8°C
SMILES 字串
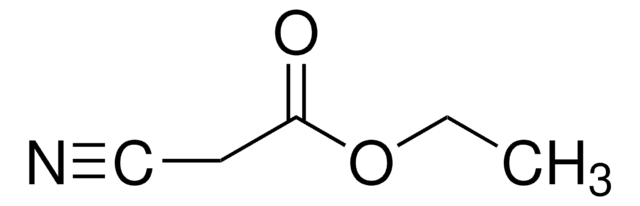
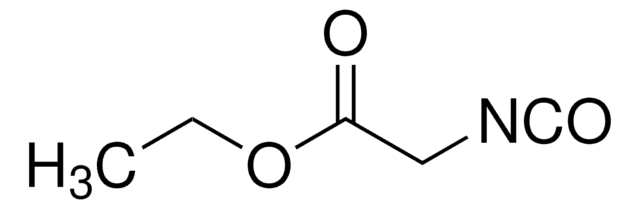
CCOC(=O)C[N+]#[C-]
InChI
1S/C5H7NO2/c1-3-8-5(7)4-6-2/h3-4H2,1H3
InChI 密鑰
FPULFENIJDPZBX-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
异氰酸乙酯是一种异氰酸酯,是生产杂环的合成砌块。
應用
异氰基乙酸乙酯被用于合成7-氮杂-四氢吲哚。它还被用于制备吡咯、噁唑啉、苯二氮卓类、噁唑和咪唑。
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
183.2 °F - closed cup
閃點(°C)
84 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Marcus Baumann et al.
Organic letters, 8(23), 5231-5234 (2006-11-03)
[Structure: see text] A multipurpose mesofluidic flow reactor capable of producing gram quantities of material has been developed as an automated synthesis platform for the rapid on-demand synthesis of key building blocks and small exploratory libraries. The reactor is configured
Yifei Li et al.
Chemical communications (Cambridge, England), 48(100), 12228-12230 (2012-11-13)
A novel and efficient route for the synthesis of 7-aza-tetrahydroindoles from N-aryl/alkyl-alkenoylacetamides and ethyl isocyanoacetate is described. A mechanism, involving a stepwise [3+2] cycloaddition-intramolecular aza-Michael addition cascade, is proposed that explains the origin of the double nucleophilic attack on the
Chikashi Kanazawa et al.
Journal of the American Chemical Society, 128(33), 10662-10663 (2006-08-17)
The copper-catalyzed reaction between two different isocyanides produces the corresponding heteroaromatization products, imidazoles, in good yields. The reaction proceeds most probably through the activation of the sp3 C-H bond in the isocyanides by a copper catalyst, followed by a [3
Z Q Gu et al.
Journal of medicinal chemistry, 36(8), 1001-1006 (1993-04-16)
A series of imidazo[1,5-a][1,4]benzodiazepine esters have been synthesized with varying ester side chains and 8-position substituents. The affinities of these compounds were evaluated at both "diazepam-insensitive" (DI) and diazepam-sensitive (DS) subtypes of the benzodiazepine receptor (BZR). A profound steric effect
Tetrahedron Letters, 47, 5481-5481 (2006)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门

![1,8-二氮杂双环[5.4.0]十一碳-7-烯 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)