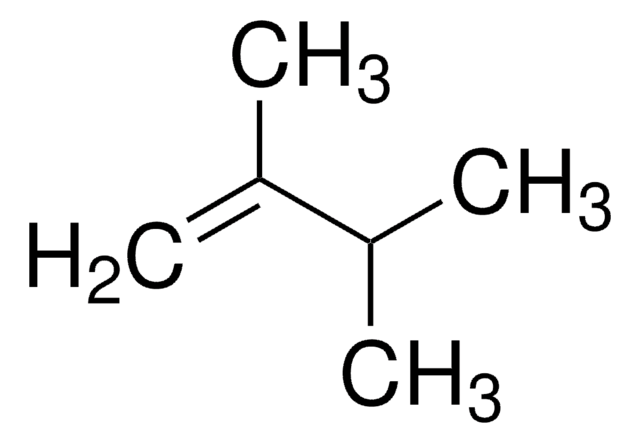
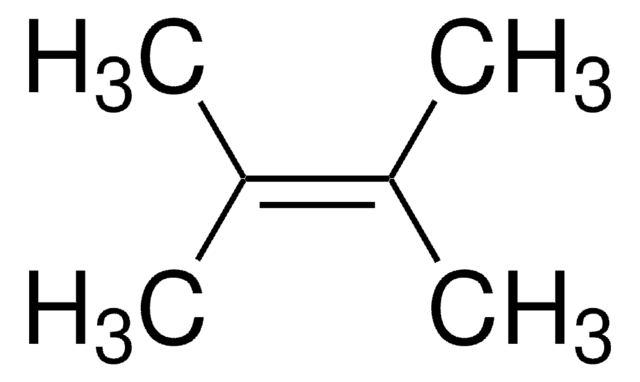
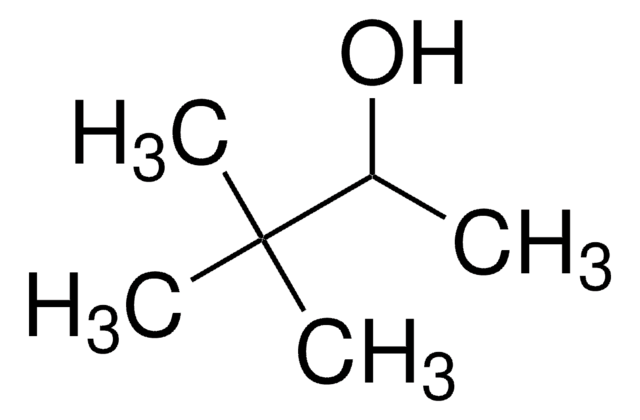
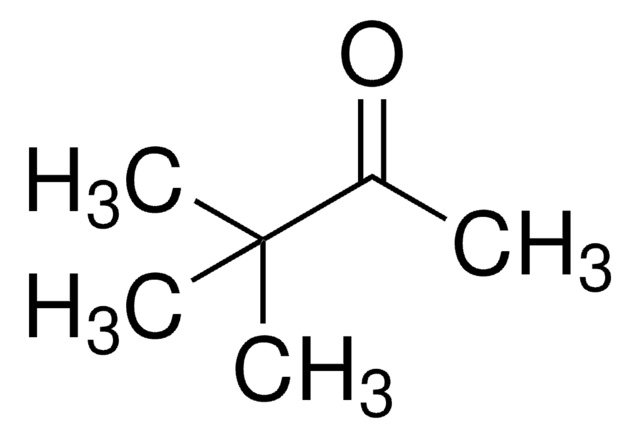
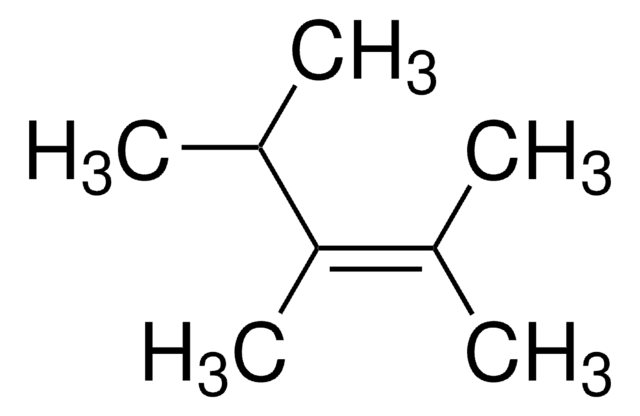
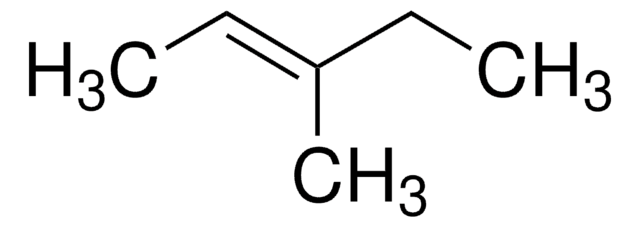
推荐产品
蒸汽壓力
215 mmHg ( 37.7 °C)
品質等級
化驗
≥99%
形狀
liquid
自燃溫度
754 °F
折射率
n20/D 1.412 (lit.)
bp
73 °C (lit.)
mp
−75 °C (lit.)
密度
0.708 g/mL at 25 °C (lit.)
儲存溫度
2-8°C
SMILES 字串
C\C(C)=C(\C)C
InChI
1S/C6H12/c1-5(2)6(3)4/h1-4H3
InChI 密鑰
WGLLSSPDPJPLOR-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
2,3-二甲基-2-丁烯在黑暗中臭氧分解产生羟基自由基 。用流动管接口式紫外光电子能谱仪研究了臭氧与 2,3-二甲基-2-丁烯 (DMB) 的反应 。在 0°C 和-15°C 下,DMB 与四氟硼酸硫代阳离子自由基形成加合物 。
應用
2,3-二甲基-2-丁烯作为底物参与了 2-羟基-1,4-萘醌的光诱导分子转化 。
訊號詞
Danger
危險聲明
危險分類
Asp. Tox. 1 - Flam. Liq. 2
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
17.6 °F - closed cup
閃點(°C)
-8 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
The Journal of Organic Chemistry, 58, 4614-4614 (1993)
Bing-Jun Zhao et al.
The Journal of organic chemistry, 71(10), 3737-3742 (2006-05-06)
Thianthrene cation radical tetrafluoroborate (Th*+ BF4-) has been found to add to 2,3-dimethyl-2-butene (DMB) at 0 degrees C and -15 degrees C. The adduct, 2,3-dimethyl-2,3-(5,10-thianthreniumdiyl)butane ditetrafluoroborate (12), was isolated at -15 degrees C, and its 1H NMR spectrum was recorded
Maryline Pflieger et al.
Environmental science & technology, 47(12), 6239-6246 (2013-05-15)
In order to investigate the heterogeneous oxidation kinetics of the herbicide terbuthylazine (TERB), a stable and reproducible generation system of "dark" hydroxyl radical in the gas phase was developed and optimized using a PTR-MS. TERB was adsorbed on silica particles
L R Pohl et al.
Biochemical and biophysical research communications, 117(2), 367-371 (1983-12-16)
Although indirect evidence has suggested that liver microsomal cytochrome P-450 can reductively dehalogenate several compounds to carbene metabolites, there has been no direct proof for the formation of these reactive species. We report in this paper that carbenes can be
R Tolando et al.
Xenobiotica; the fate of foreign compounds in biological systems, 26(4), 425-435 (1996-04-01)
1. During anaerobic reductive incubation of liver microsomes, from either the pyridine- or phenobarbital-treated rat, with 1,1-dichloro-1-fluoroethane (HCFC-141b) in the presence of a NADPH-regenerating system, a time- and dose-dependent formation of reactive metabolites was detected as indicated by a depletion
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门