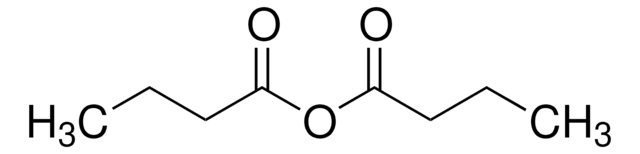
推荐产品
品質等級
化驗
99%
形狀
liquid
折射率
n20/D 1.409 (lit.)
bp
193 °C (lit.)
密度
0.918 g/mL at 25 °C (lit.)
官能基
anhydride
ester
SMILES 字串
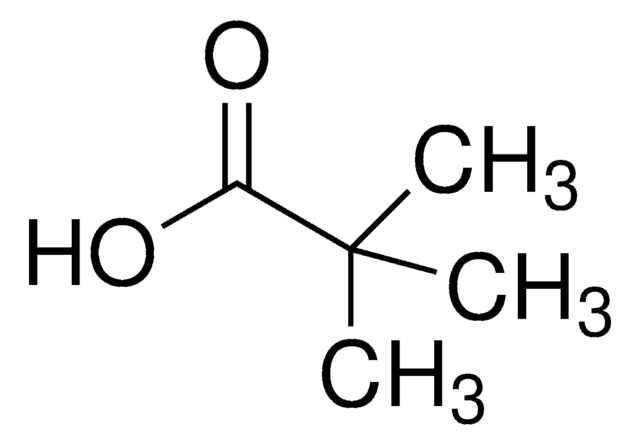
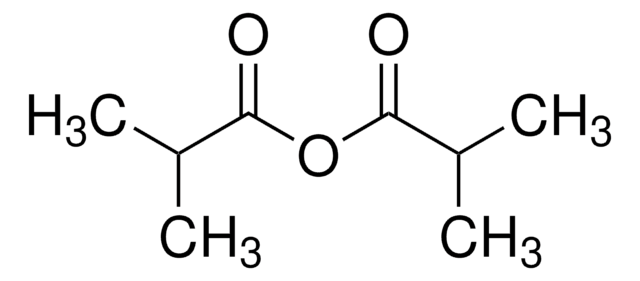
CC(C)(C)C(=O)OC(=O)C(C)(C)C
InChI
1S/C10H18O3/c1-9(2,3)7(11)13-8(12)10(4,5)6/h1-6H3
InChI 密鑰
PGZVFRAEAAXREB-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
三甲基乙酸酐被用于:
- 固相寡核苷酸合成
- 外消旋 2-羟基-γ-动力学拆分-丁内酯与二苯乙酸
- 作为苯胺的酰化和酯化试剂
- 作为酚类的酰化和酯化试剂
分别为苯胺和酚的酰化和酯化试剂。
訊號詞
Warning
危險分類
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
162.5 °F - closed cup
閃點(°C)
72.5 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
其他客户在看
Alexey Evdokimov et al.
Nucleic acids research, 41(12), e123-e123 (2013-04-24)
DNA probes for the studies of damaged strand excision during the nucleotide excision repair (NER) have been designed using the novel non-nucleosidic phosphoramidite reagents that contain N-[6-(9-antracenylcarbamoyl)hexanoyl]-3-amino-1,2-propandiol (nAnt) and N-[6-(5(6)-fluoresceinylcarbamoyl)hexanoyl]-3-amino-1,2-propandiol (nFlu) moieties. New lesion-imitating adducts being inserted into DNA show
Bulletin of the Chemical Society of Japan, 67, 210-210 (1994)
Australian Journal of Chemistry, 60, 75-75 (2007)
Z J Kamiński
International journal of peptide and protein research, 43(3), 312-319 (1994-03-01)
According to the concept presented, esters forming an amide (peptide) bond by the mechanism SN#DN or SN#*DN involving fast decay of the tetrahedral intermediate may behave as 'superactive acylating reagents'. These should render coupling involving less reactive substrates, i.e. sterically
Q Zhu et al.
Bioorganic & medicinal chemistry letters, 11(9), 1105-1107 (2001-05-17)
Commercially available 'fast-deprotecting' phosphoramidites are useful for synthesizing oligonucleotides containing alkali-sensitive nucleotides. However, N-acetylated oligonucleotides were observed during solid-phase synthesis using 'fast-deprotecting' phosphoramidites in conjunction with K2CO3/MeOH ('ultra-mild') deprotection. Transamidation was localized at deoxyguanosine, which is protected as its isopropylphenoxyacetyl
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门