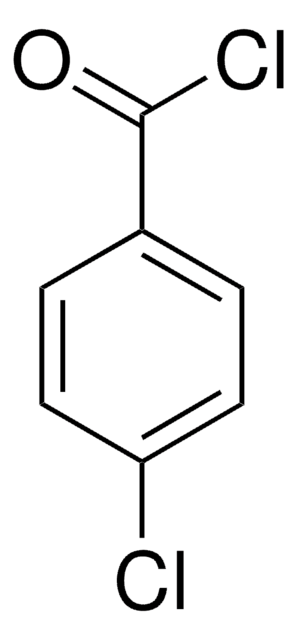
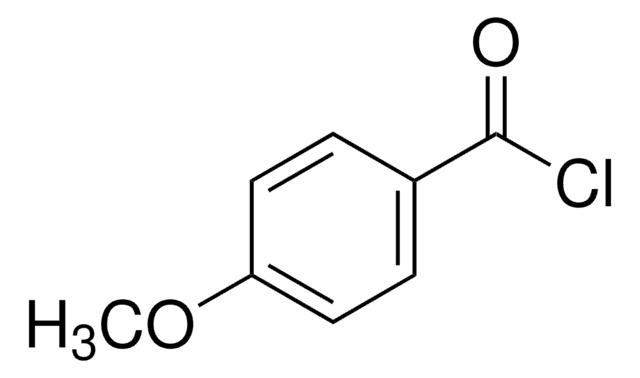
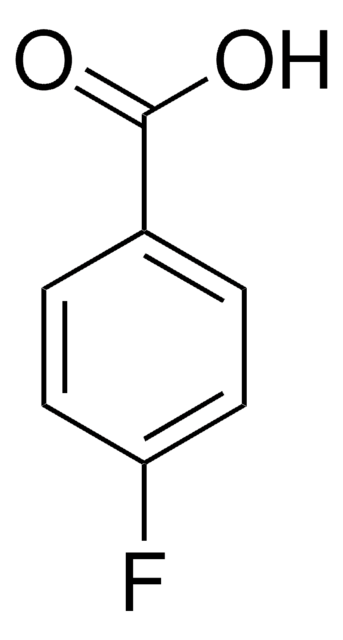
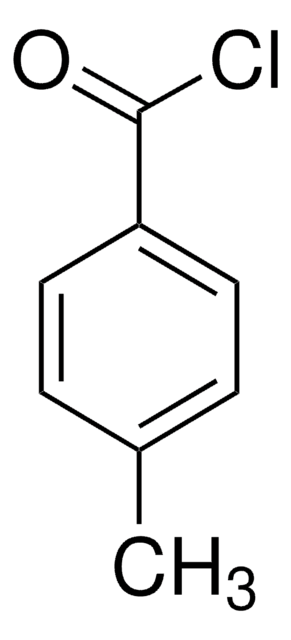
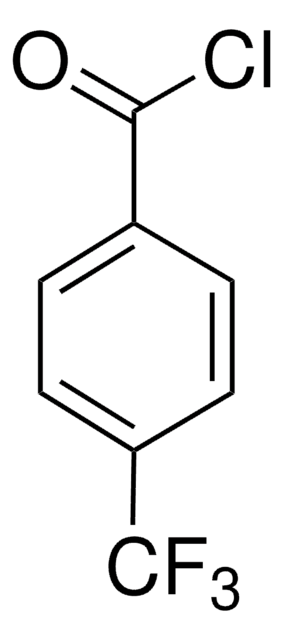
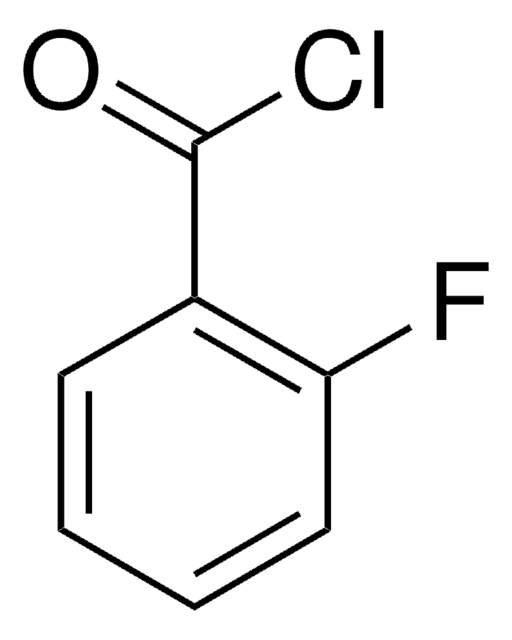
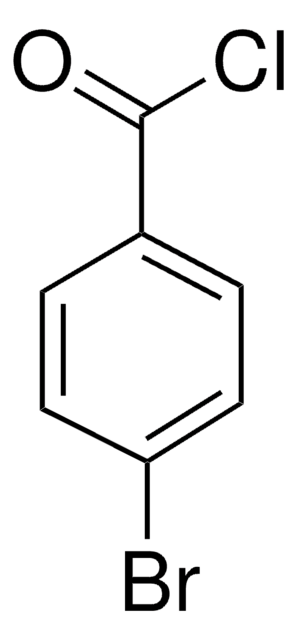
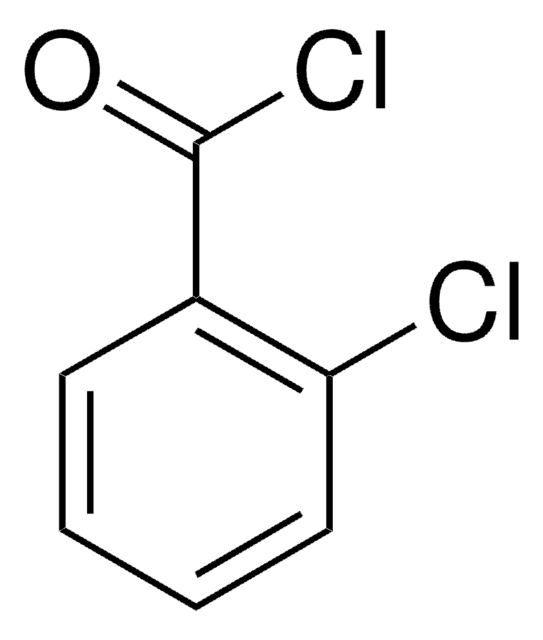
推荐产品
化驗
98%
折射率
n20/D 1.532 (lit.)
bp
82 °C/20 mmHg (lit.)
mp
10-12 °C (lit.)
密度
1.342 g/mL at 25 °C (lit.)
官能基
acyl chloride
SMILES 字串
Fc1ccc(cc1)C(Cl)=O
InChI
1S/C7H4ClFO/c8-7(10)5-1-3-6(9)4-2-5/h1-4H
InChI 密鑰
CZKLEJHVLCMVQR-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
訊號詞
Danger
危險聲明
危險分類
Skin Corr. 1B
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
179.6 °F - closed cup
閃點(°C)
82 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
其他客户在看
商品
The Friedel–Crafts acylation is the reaction of an arene with acyl chlorides or anhydrides using a strong Lewis acid catalyst. This reaction proceeds via electrophilic aromatic substitution to form monoacylated products.
The Friedel–Crafts acylation is the reaction of an arene with acyl chlorides or anhydrides using a strong Lewis acid catalyst. This reaction proceeds via electrophilic aromatic substitution to form monoacylated products.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门