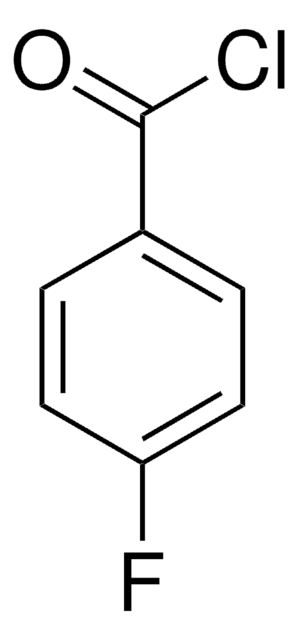
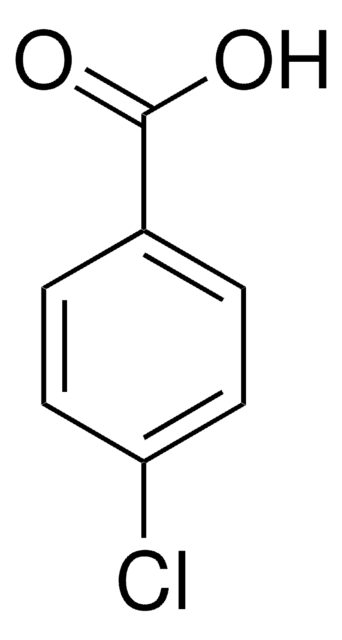
推荐产品
化驗
99%
折射率
n20/D 1.578 (lit.)
bp
102-104 °C/11 mmHg (lit.)
mp
11-14 °C (lit.)
密度
1.365 g/mL at 20 °C (lit.)
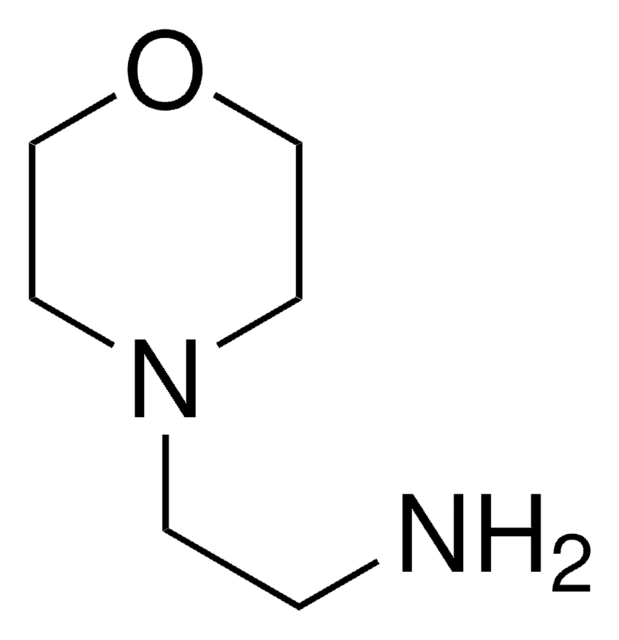
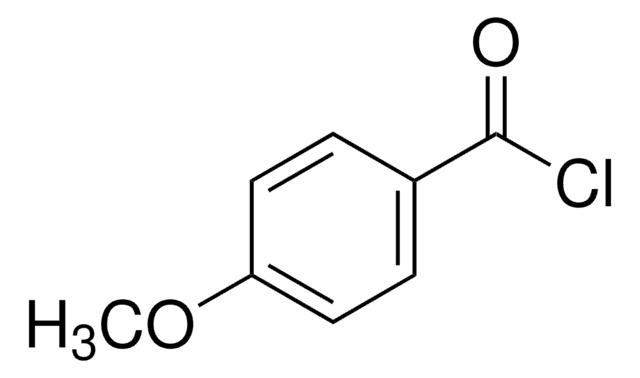
SMILES 字串
ClC(=O)c1ccc(Cl)cc1
InChI
1S/C7H4Cl2O/c8-6-3-1-5(2-4-6)7(9)10/h1-4H
InChI 密鑰
RKIDDEGICSMIJA-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
4-氯苯甲酰氯可用作药物合成中的中间化合物。
應用
4-氯苯甲酰氯在 KHCO3 缓冲液中与 CoA 反应合成 4-氯苯甲酰 CoA 。
訊號詞
Danger
危險聲明
危險分類
Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1B
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 1
閃點(°F)
221.0 °F - closed cup
閃點(°C)
105 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
其他客户在看
S D Copley et al.
Applied and environmental microbiology, 58(4), 1385-1387 (1992-04-01)
4-Chlorobenzoate degradation in cell extracts of Acinetobacter sp. strain 4-CB1 occurs by initial synthesis of 4-chlorobenzoyl coenzyme A (4-chlorobenzoyl CoA) from 4-chlorobenzoate, CoA, and ATP. 4-Chlorobenzoyl CoA is dehalogenated to 4-hydroxybenzoyl CoA. Following the dehalogenation reaction, 4-hydroxybenzoyl CoA is hydrolyzed
Iteb Trabelsi et al.
Journal of oleo science, 66(7), 667-676 (2017-07-05)
The article deals with the use of mixed anhydrides for the synthesis of fatty esters. Both aliphatic and aromatic acids are involved, indicating different behaviors according to the chain length of the aliphatic acid. We describe a novel and efficient
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门