推荐产品
產品線
ReagentPlus®
化驗
99%
bp
170-172 °C/10 mmHg (lit.)
mp
105-107 °C (lit.)
溶解度
95% ethanol: soluble 50 mg/mL, clear, colorless to faintly yellow
官能基
carboxylic acid
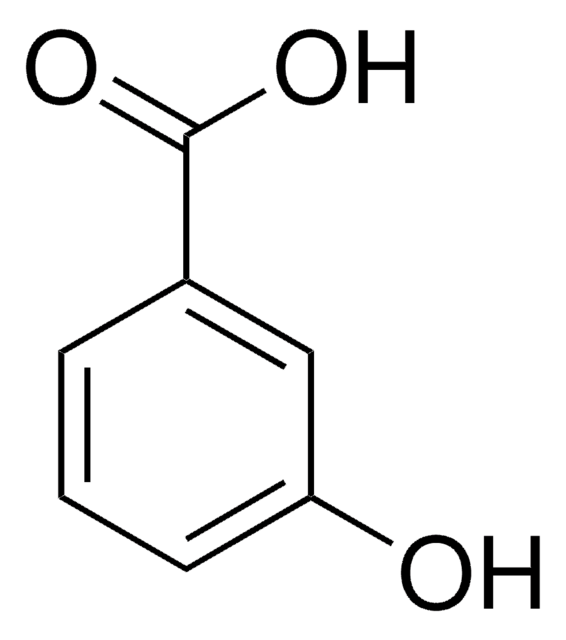
SMILES 字串
COc1cccc(c1)C(O)=O
InChI
1S/C8H8O3/c1-11-7-4-2-3-6(5-7)8(9)10/h2-5H,1H3,(H,9,10)
InChI 密鑰
XHQZJYCNDZAGLW-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
3-甲氧基苯甲酸是天然产物合成中的重要中间体。
應用
3-甲氧基苯甲酸用于合成和表征铕(III)和钆(III)的 3-甲氧基苯甲酸酯。将其用于将芳族羧酸转化为甲酯并使用硼氢化钠-THF-甲醇系统还原成相应的伯醇。
法律資訊
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Synthesis, characterization and thermal behaviour of solid-state compounds of europium (III) and gadolinium (III) 3-methoxybenzoate.
Dametto PR, et al.
Journal of Thermal Analysis and Calorimetry, 97(2), 765-768 (2009)
Sodium borohydride reduction of aromatic carboxylic acids via methyl esters.
Saeed A and Ashraf Z.
Journal of Chemical Sciences (Bangalore), 118(5), 419-423 (2006)
N Dodoff et al.
Journal of inorganic biochemistry, 54(3), 221-233 (1994-05-15)
The complexes [Pt(bah)2X2], [Pt(NH3)(bah)Cl2].0.5H2O, [Pt(mbah)2X2], and [Pt(NH3)(mbah)Cl2] (bah = benzoic acid hydrazide, mbah = 3-methoxybenzoic acid hydrazide; X = Cl, Br, I) have been prepared and characterized by elemental analysis, electric conductivity, IR, 1H NMR, and electronic spectra. A cis-square
K A DeWeerd et al.
Applied and environmental microbiology, 54(5), 1237-1242 (1988-05-01)
O-methyl substituents of aromatic compounds can provide C1 growth substrates for facultative and strict anaerobic bacteria isolated from diverse environments. The mechanism of the bioconversion of methoxylated benzoic acids to the hydroxylated derivatives was investigated with a model substrate and
Thi-Huu Nguyen et al.
Organic letters, 7(12), 2445-2448 (2005-06-04)
[reaction: see text] If employed in THF at 0 degrees C, LTMP metalates meta-anisic acid at the doubly activated position. In contrast, n-BuLi/t-BuOK deprotonates position C-4 preferentially at low temperature. Functionalization at C-6 requires protection of the C-2 site beforehand.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门






![苯并[a]芴酮 BCR®, certified reference material](/deepweb/assets/sigmaaldrich/product/structures/881/090/eae85258-97ed-4de7-90c1-c0e0e495552e/640/eae85258-97ed-4de7-90c1-c0e0e495552e.png)