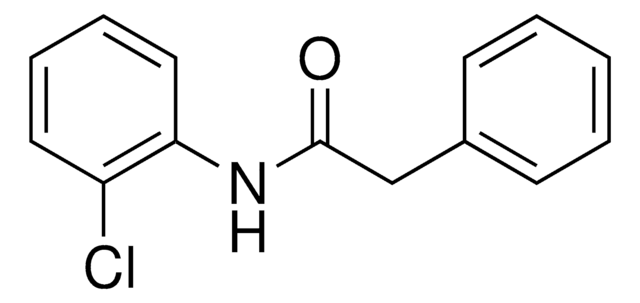
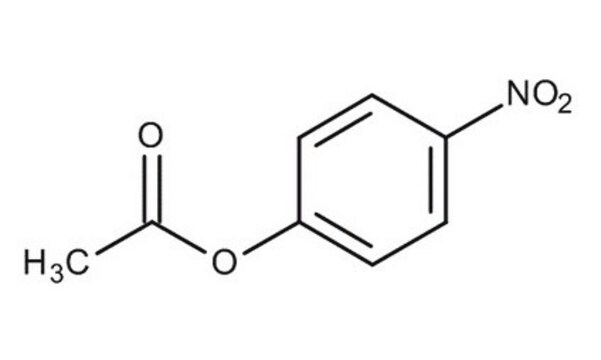
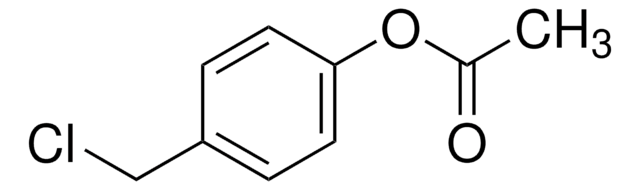
推荐产品
品質等級
化驗
99%
折射率
n20/D 1.501 (lit.)
bp
196 °C (lit.)
密度
1.073 g/mL at 25 °C (lit.)
官能基
ester
phenoxy
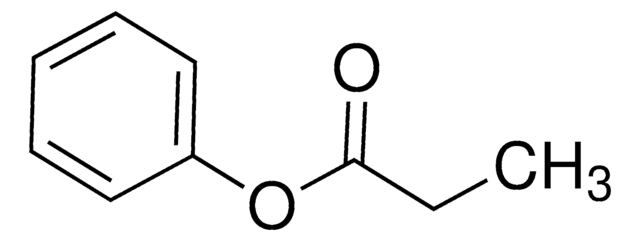
SMILES 字串
CC(=O)Oc1ccccc1
InChI
1S/C8H8O2/c1-7(9)10-8-5-3-2-4-6-8/h2-6H,1H3
InChI 密鑰
IPBVNPXQWQGGJP-UHFFFAOYSA-N
基因資訊
human ... PON1(5444)
正在寻找类似产品? 访问 产品对比指南
一般說明
其他客户在看
商品
The Fries rearrangement reaction is an organic name reaction which involves the conversion of phenolic esters into hydroxyaryl ketones on heating in the presence of a catalyst. Suitable catalysts for this reaction are Brønsted or Lewis acids such as HF, AlCl3, BF3, TiCl4, or SnCl4. The Fries rearrangement reaction is an ortho, para-selective reaction, and is used in the preparation of acyl phenols. This organic reaction has been named after German chemist Karl Theophil Fries.
The Fries rearrangement reaction is an organic name reaction which involves the conversion of phenolic esters into hydroxyaryl ketones on heating in the presence of a catalyst. Suitable catalysts for this reaction are Brønsted or Lewis acids such as HF, AlCl3, BF3, TiCl4, or SnCl4. The Fries rearrangement reaction is an ortho, para-selective reaction, and is used in the preparation of acyl phenols. This organic reaction has been named after German chemist Karl Theophil Fries.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门