912654
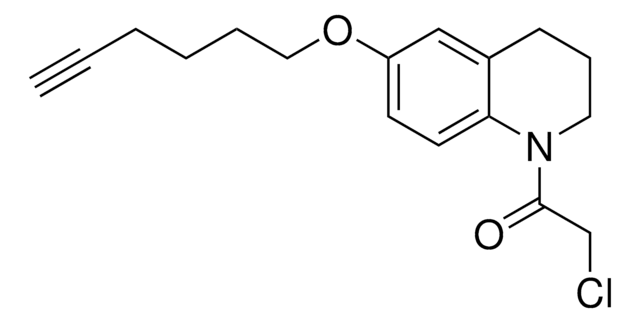
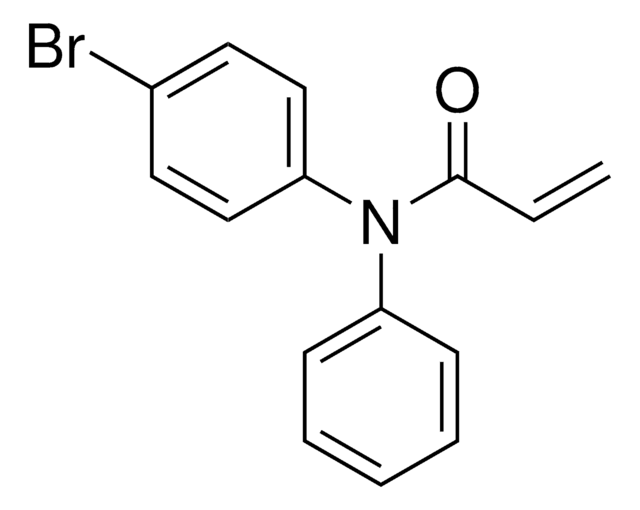
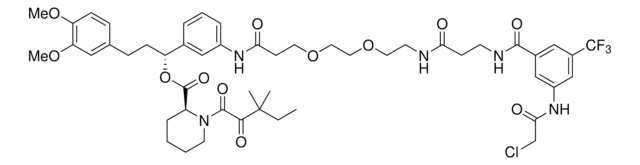
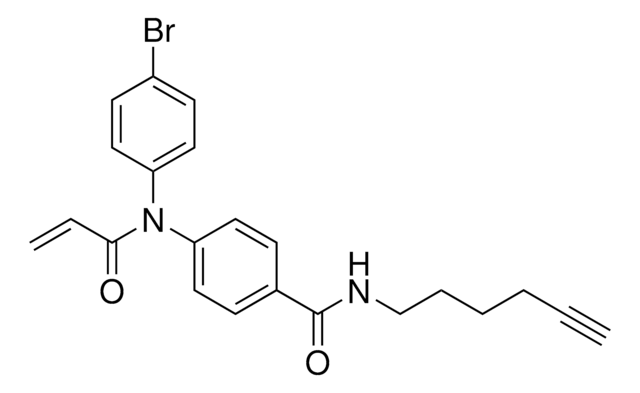
N-(3,5-Bis(trifluoromethyl)phenyl)-2-chloroacetamide
≥95%
Synonyme(s) :
N-Chloroacetyl-3,5-bis(trifluoromethyl)aniline, Electrophilic scout fragment, KB03, Scout fragment for targetable cysteine
About This Item
Produits recommandés
Pureté
≥95%
Forme
powder
InChI
1S/C10H6ClF6NO/c11-4-8(19)18-7-2-5(9(12,13)14)1-6(3-7)10(15,16)17/h1-3H,4H2,(H,18,19)
Clé InChI
LEYIUTOAQOUAFG-UHFFFAOYSA-N
Application
Autres remarques
Informations légales
Produit(s) apparenté(s)
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Skin Irrit. 2
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Vous ne trouvez pas la bonne version ?
Si vous avez besoin d'une version particulière, vous pouvez rechercher un certificat spécifique par le numéro de lot.
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Articles
Ligandability describes the propensity of a protein target to bind a small molecule with high affinity. It is a precursor to evaluating druggability, which requires more advanced translational pharmacological effects and drug-like properties in vivo.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique