A78403
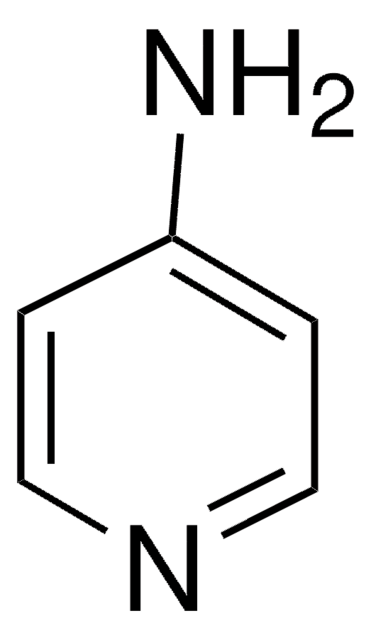
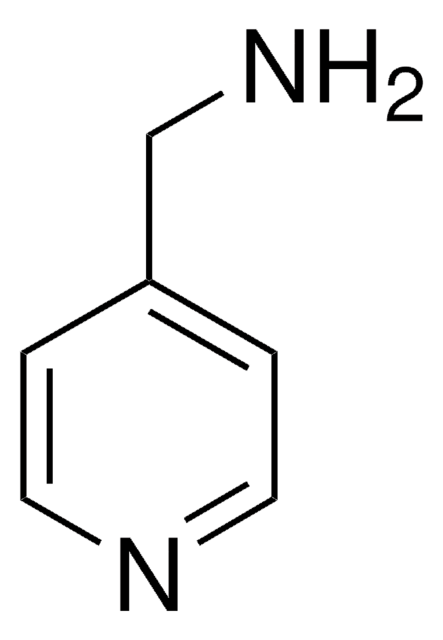
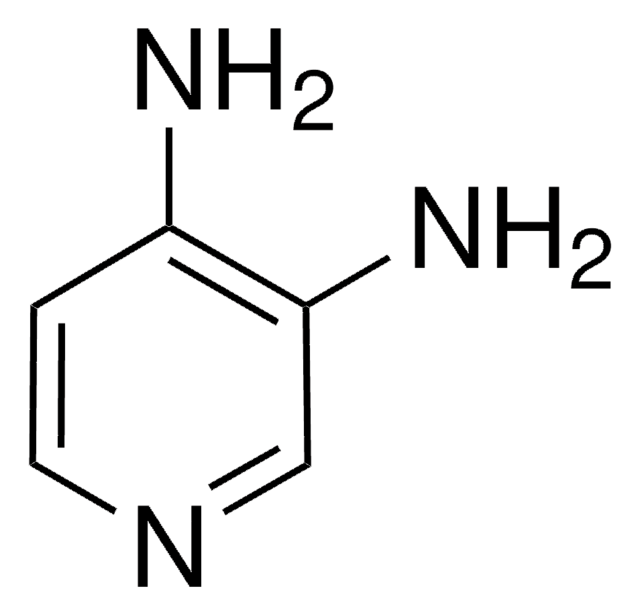
4-Aminopyridine
98%
Synonym(s):
4-Pyridinamine, 4-Pyridylamine, 4AP, Fampridine
About This Item
Recommended Products
Quality Level
Assay
98%
form
crystals
bp
273 °C (lit.)
mp
155-158 °C (lit.)
solubility
H2O: 50 mg/mL, clear, colorless
SMILES string
Nc1ccncc1
InChI
1S/C5H6N2/c6-5-1-3-7-4-2-5/h1-4H,(H2,6,7)
InChI key
NUKYPUAOHBNCPY-UHFFFAOYSA-N
Gene Information
human ... KCNA1(3736) , KCNA10(3744) , KCNA2(3737) , KCNA3(3738) , KCNA4(3739) , KCNA5(3741) , KCNA6(3742) , KCNA7(3743) , KCNB1(3745) , KCNB2(9312) , KCNC1(3746) , KCNC2(3747) , KCNC3(3748) , KCNC4(3749) , KCND1(3750) , KCND2(3751) , KCND3(3752) , KCNF1(3754) , KCNG1(3755) , KCNG2(26251) , KCNG3(170850) , KCNG4(93107) , KCNH1(3756) , KCNH2(3757) , KCNH3(23416) , KCNH4(23415) , KCNH5(27133) , KCNH6(81033) , KCNH7(90134) , KCNH8(131096) , KCNQ1(3784) , KCNQ2(3785) , KCNQ3(3786) , KCNQ4(9132) , KCNQ5(56479) , KCNS1(3787) , KCNS2(3788) , KCNS3(3790) , KCNV1(27012) , KCNV2(169522)
mouse ... Kcna3(16491)
rat ... Grin2a(24409)
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Catalyst in the regioselective acylation of N-tosylhydrazide.
- Starting material in the synthesis of 4-aminopyridine derivatives for neurological disorder studies.
- Precursor for the synthesis of enantiomerically pure 4-(pyrrolidino)pyridine (PPY) derivatives by cyclocondensation reaction.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Oral - Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Aquatic Chronic 2 - Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
312.8 °F
Flash Point(C)
156 °C
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Global Trade Item Number
| SKU | GTIN |
|---|---|
| A78403-100G | 4061833386491 |
| A78403-1KG | |
| A78403-25G | 4061833386507 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service