About This Item
Productos recomendados
Formulario
powder
Nivel de calidad
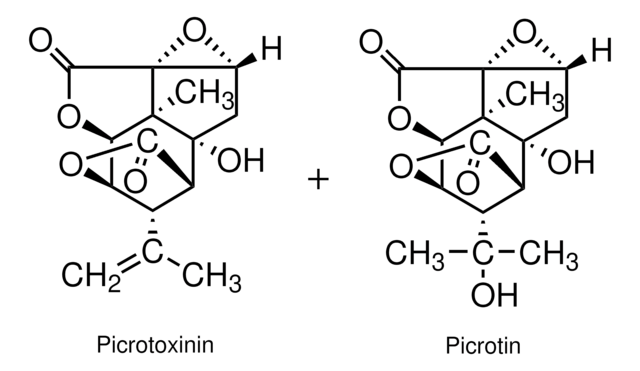
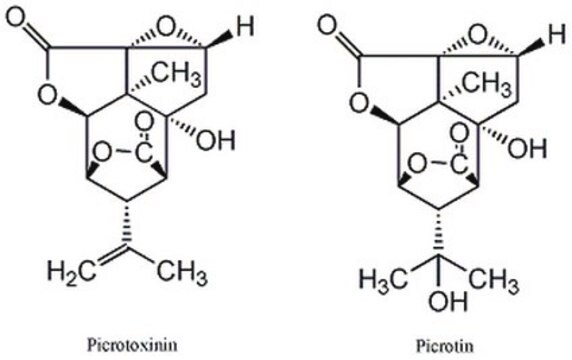
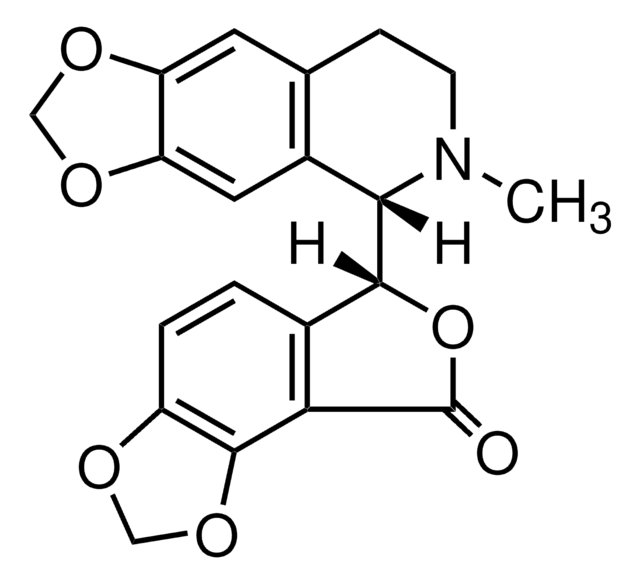
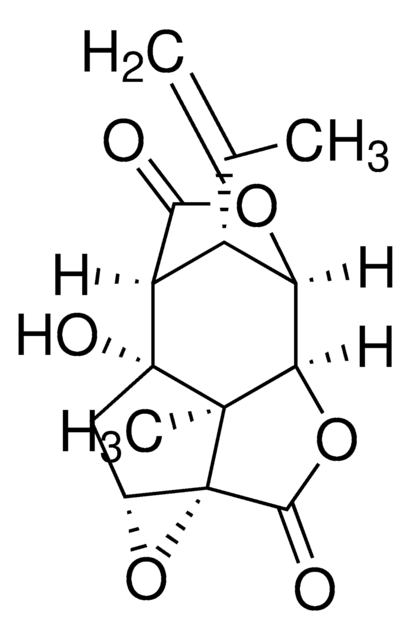
cadena SMILES
[H][C@@]12OC(=O)[C@]([H])([C@@H]1C(C)=C)[C@]3(O)C[C@H]4O[C@]45C(=O)O[C@@]2([H])[C@]35C
InChI
1S/C15H16O6/c1-5(2)7-8-11(16)19-9(7)10-13(3)14(8,18)4-6-15(13,21-6)12(17)20-10/h6-10,18H,1,4H2,2-3H3/t6-,7+,8+,9-,10-,13-,14-,15+/m1/s1
Clave InChI
PIMZUZSSNYHVCU-KBLUICEQSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Aplicación
Acciones bioquímicas o fisiológicas
Características y beneficios
Otras notas
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 2 Oral
Código de clase de almacenamiento
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico