D1692
GW4869
≥90% (NMR), powder, N-SMase inhibitor
Sinónimos:
N,N′-Bis[4-(4,5-dihydro-1H-imidazol-2-yl)phenyl]-3,3′-p-phenylene-bis-acrylamide dihydrochloride
About This Item
Productos recomendados
Nombre del producto
GW4869, ≥90% (NMR)
Nivel de calidad
Ensayo
≥90% (NMR)
Formulario
powder
condiciones de almacenamiento
desiccated
protect from light
color
light yellow to yellow
mp
>300 °C
solubilidad
DMSO: 0.2 mg/mL
emisor
GlaxoSmithKline
temp. de almacenamiento
2-8°C
cadena SMILES
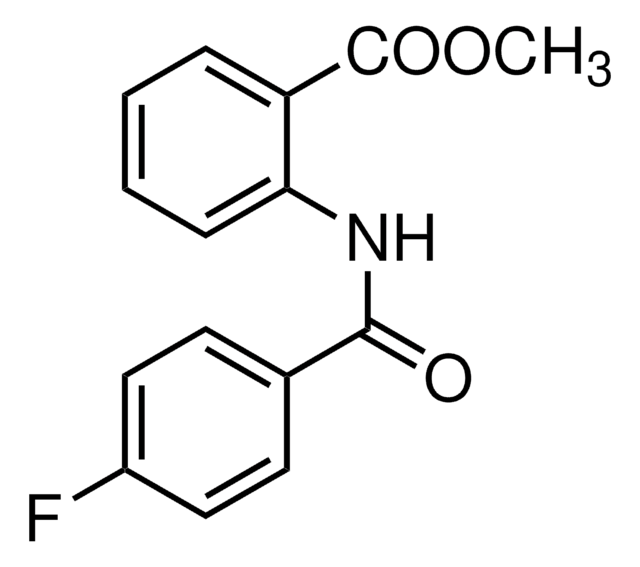
Cl.Cl.O=C(Nc1ccc(cc1)C2=NCCN2)\C=C/c3ccc(\C=C/C(=O)Nc4ccc(cc4)C5=NCCN5)cc3
InChI
1S/C30H28N6O2.2ClH/c37-27(35-25-11-7-23(8-12-25)29-31-17-18-32-29)15-5-21-1-2-22(4-3-21)6-16-28(38)36-26-13-9-24(10-14-26)30-33-19-20-34-30;;/h1-16H,17-20H2,(H,31,32)(H,33,34)(H,35,37)(H,36,38);2*1H/b15-5-,16-6-;;
Clave InChI
NSFKAZDTKIKLKT-LOLTXFFGSA-N
Descripción general
Aplicación
- as an inhibitor of neutral sphingomyelinase and exosome biogenesis
- to analyse the effects of arsenic trioxide (ATO) treatment for hepatoma carcinoma HCCLM3 cells on ceramide production
- to determine the contributions of p75 neurotrophin receptor (p75NTR) and tropomyosin receptor kinase A (TrkA)- coupled pathways to nerve growth factor (NGF)-induced thermal hypersensitivity in rats
Acciones bioquímicas o fisiológicas
Características y beneficios
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico