W291102
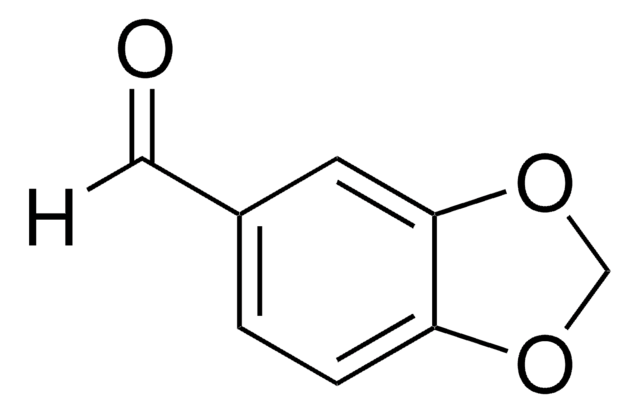
Piperonal
≥99%, FCC, FG
Sinónimos:
1,3-Benzodioxole-5-carboxaldehyde, 3,4-(Methylenedioxy)benzaldehyde, Heliotropin
About This Item
Fragrance grade
Halal
Kosher
meets purity specifications of JECFA
Productos recomendados
origen biológico
synthetic
Nivel de calidad
grado
FG
Fragrance grade
Halal
Kosher
Agency
follows IFRA guidelines
meets purity specifications of JECFA
cumplimiento norm.
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
FCC
FDA 21 CFR 182.60
presión de vapor
1 mmHg ( 87 °C)
Ensayo
≥99%
bp
264 °C (lit.)
mp
35-39 °C (lit.)
aplicaciones
flavors and fragrances
Documentación
see Safety & Documentation for available documents
alérgeno alimentario
no known allergens
alérgeno de la fragancia
heliotropine
Organoléptico
cherry; sweet; vanilla
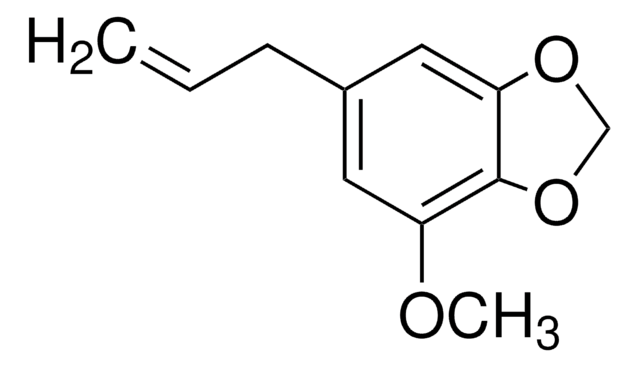
cadena SMILES
[H]C(=O)c1ccc2OCOc2c1
InChI
1S/C8H6O3/c9-4-6-1-2-7-8(3-6)11-5-10-7/h1-4H,5H2
Clave InChI
SATCULPHIDQDRE-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Aplicación
- The synthesis and characterisation of MDMA derived from a catalytic oxidation of material isolated from black pepper reveals potential route specific impurities.: This study explores the synthesis and characterization of MDMA from piperonal, highlighting potential impurities unique to this synthesis route. This research has implications for forensic science and the identification of synthetic routes for MDMA (Plummer et al., 2016).
- Design, synthesis, and biological evaluation of platensimycin analogues with varying degrees of molecular complexity.: This paper details the synthesis of platensimycin analogues using piperonal derivatives. The study evaluates the biological activities of these analogues, contributing to the development of new antibacterial agents (Nicolaou et al., 2008).
- Synthesis and use of 4-peptidylhydrazido-N-hexyl-1,8-naphthalimides as fluorogenic histochemical substrates for dipeptidyl peptidase IV and tripeptidyl peptidase I.: This research presents the synthesis of piperonal-based substrates for histochemical applications, enabling the study of enzyme activities in biochemical assays (Ivanov et al., 2009).
Acciones bioquímicas o fisiológicas
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Skin Sens. 1
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 2
Punto de inflamabilidad (°F)
150.1 °F
Punto de inflamabilidad (°C)
65.62 °C
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico