268909
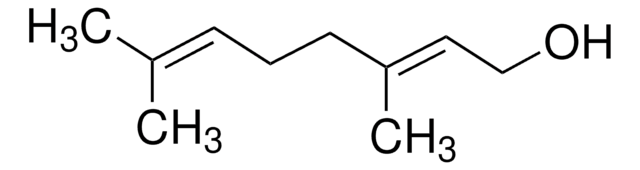
cis-3,7-Dimethyl-2,6-octadien-1-ol
97%
Sinónimos:
Nerol
About This Item
Productos recomendados
Nivel de calidad
Ensayo
97%
Formulario
liquid
índice de refracción
n20/D 1.474 (lit.)
bp
103-105 °C/9 mmHg (lit.)
solubilidad
absolute ethanol: soluble(lit.)
densidad
0.876 g/mL at 25 °C (lit.)
grupo funcional
hydroxyl
cadena SMILES
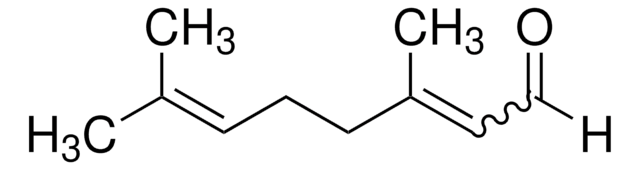
C\C(C)=C\CC\C(C)=C/CO
InChI
1S/C10H18O/c1-9(2)5-4-6-10(3)7-8-11/h5,7,11H,4,6,8H2,1-3H3/b10-7-
Clave InChI
GLZPCOQZEFWAFX-YFHOEESVSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Descripción general
Aplicación
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1B
Código de clase de almacenamiento
10 - Combustible liquids
Clase de riesgo para el agua (WGK)
WGK 2
Punto de inflamabilidad (°F)
226.0 °F - closed cup
Punto de inflamabilidad (°C)
107.78 °C - closed cup
Equipo de protección personal
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Protocolos
-3,7-Dimethyl-2,6-octadien-1-ol; Neral; Geraniol; Geranial; Undecanal; Citronellyl acetate; Neryl acetate; 3,7-Dimethyl-2,6-octadienyl acetate; 1-Tetradecene; Tetradecane; α-Bisabolol
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico