Wichtige Dokumente
A4487
Aphidicolin, Ready Made Solution
from Nigrospora sphaerica
Synonym(e):
ICI 69653, NSC-234714
About This Item
Empfohlene Produkte
Biologische Quelle
Nigrospora sphaerica
Qualitätsniveau
Dampfdruck
0.55 hPa ( 20 °C)
Form
liquid
Farbe
clear colorless
Löslichkeit
H2O: miscible (completely)
Wirkungsspektrum von Antibiotika
neoplastics
viruses
Wirkungsweise
DNA synthesis | interferes
enzyme | inhibits
Lagertemp.
−20°C
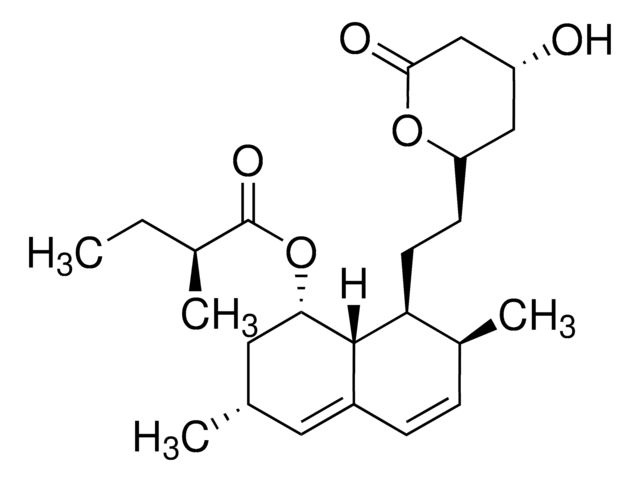
SMILES String
O[C@]1([C@H]2C[C@@]3([C@@]4([C@H]([C@@]([C@@H](CC4)O)(CO)C)CC[C@H]3C2)C)CC1)CO
InChI
1S/C20H34O4/c1-17(11-21)15-4-3-13-9-14-10-19(13,7-8-20(14,24)12-22)18(15,2)6-5-16(17)23/h13-16,21-24H,3-12H2,1-2H3/t13-,14+,15-,16+,17-,18-,19-,20-/m0/s1
InChIKey
NOFOAYPPHIUXJR-APNQCZIXSA-N
Allgemeine Beschreibung
Anwendung
Biochem./physiol. Wirkung
Verpackung
Physikalische Form
Sonstige Hinweise
Lagerklassenschlüssel
10 - Combustible liquids
WGK
WGK 1
Flammpunkt (°F)
188.6 °F - closed cup
Flammpunkt (°C)
87 °C - closed cup
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.