Wichtige Dokumente
17944
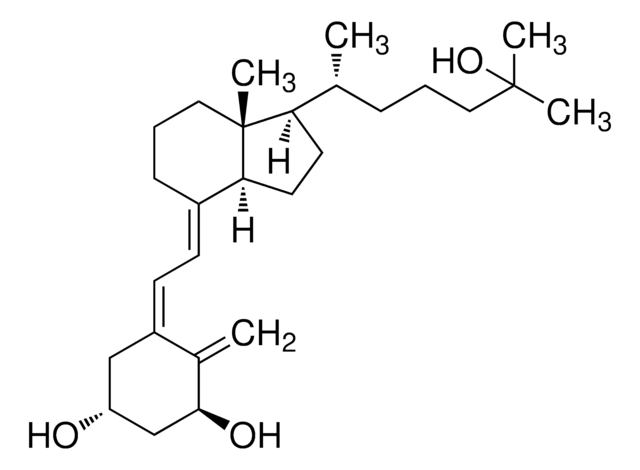
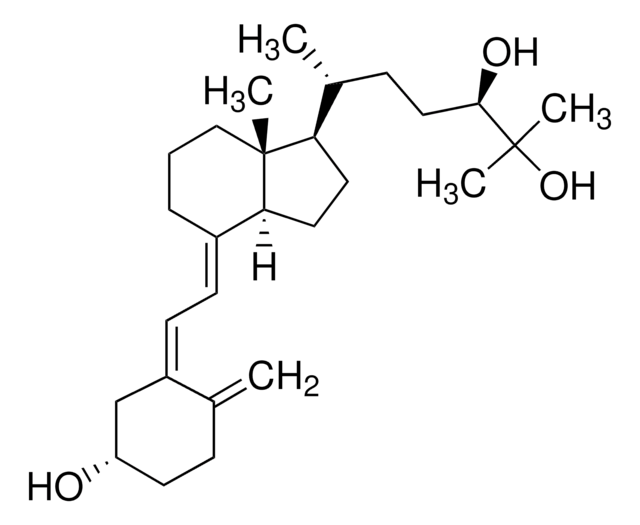
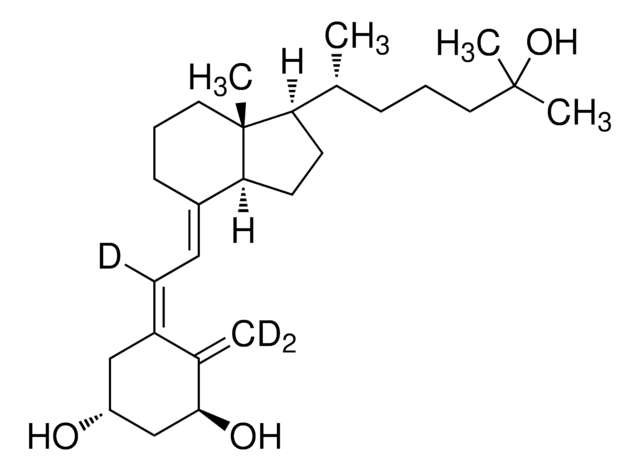
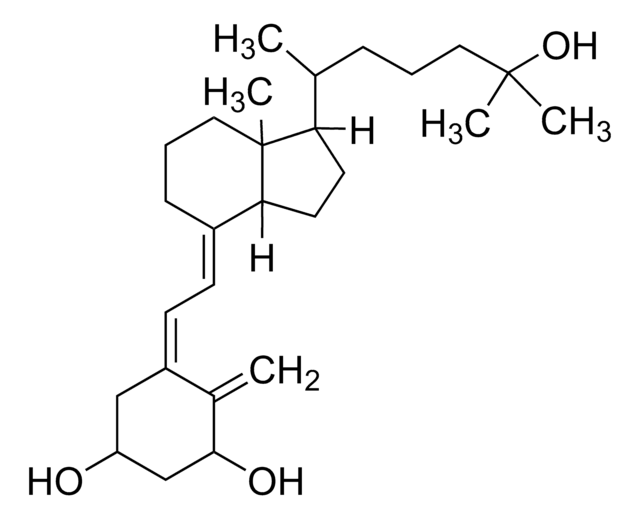
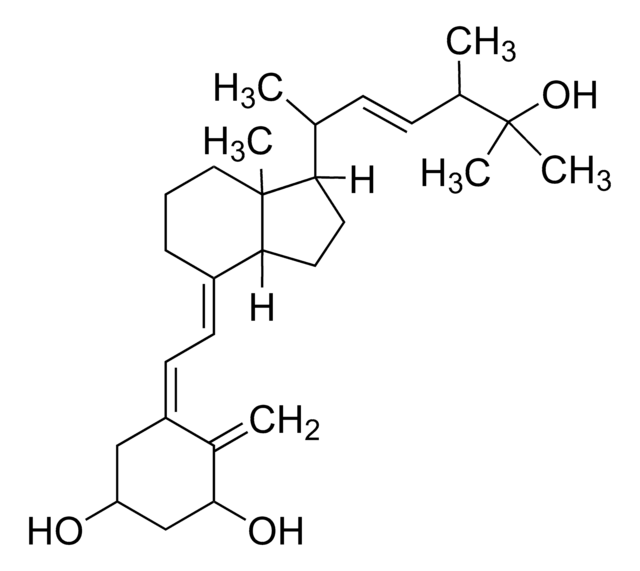
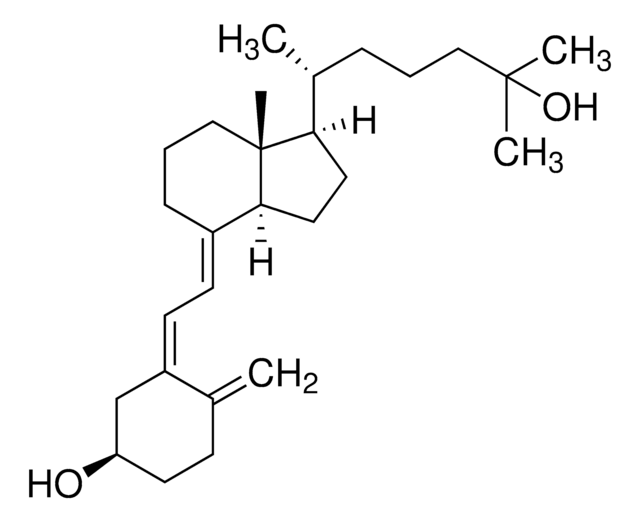
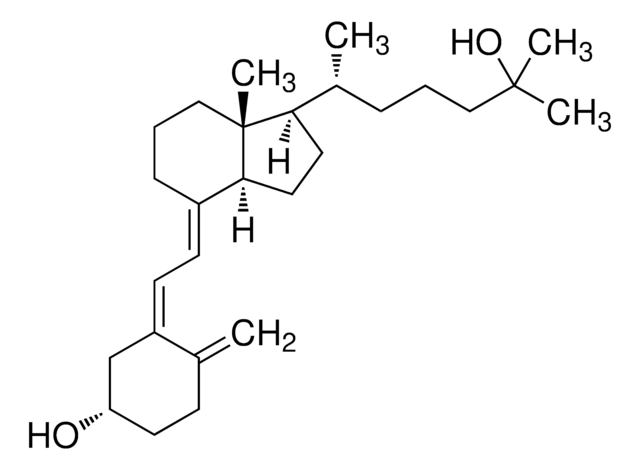
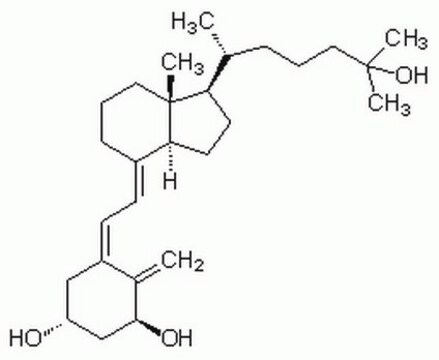
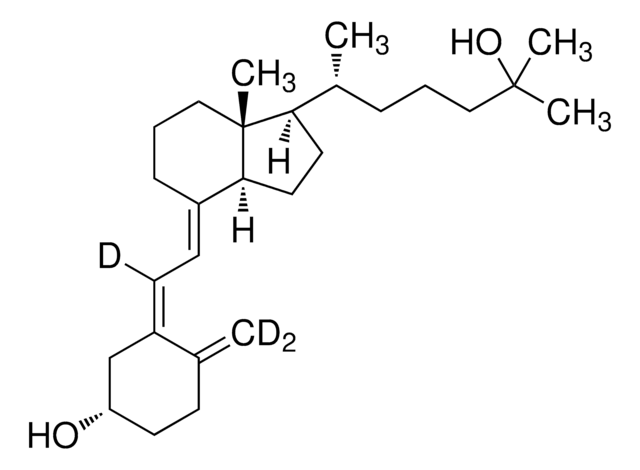
1α,25-Dihydroxyvitamin D2
≥97.0% (sum of vitamin and previtamin, HPLC)
Synonym(e):
1α,25-Dihydroxy-calciferol
About This Item
Empfohlene Produkte
Biologische Quelle
synthetic
Assay
≥97.0% (sum of vitamin and previtamin, HPLC)
Form
crystals
Farbe
white to light yellow
Lagertemp.
−70°C
SMILES String
C[C@H](\C=C\[C@H](C)C(C)(C)O)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C3\C[C@@H](O)C[C@H](O)C3=C
InChI
1S/C28H44O3/c1-18(9-10-19(2)27(4,5)31)24-13-14-25-21(8-7-15-28(24,25)6)11-12-22-16-23(29)17-26(30)20(22)3/h9-12,18-19,23-26,29-31H,3,7-8,13-17H2,1-2,4-6H3/b10-9+,21-11+,22-12-/t18-,19+,23-,24-,25+,26+,28-/m1/s1
InChIKey
ZGLHBRQAEXKACO-XJRQOBMKSA-N
Biochem./physiol. Wirkung
Verpackung
Ähnliches Produkt
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Acute Tox. 2 Inhalation - Acute Tox. 3 Dermal - Acute Tox. 3 Oral - STOT RE 1 Oral
Lagerklassenschlüssel
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
Die passende Version wird nicht angezeigt?
Wenn Sie eine bestimmte Version benötigen, können Sie anhand der Lot- oder Chargennummer nach einem spezifischen Zertifikat suchen.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
Vitamin D2 (ergocalciferol) is naturally synthesized from ergosterol by invertebrates, fungi, and plants in response to ultraviolet B irradiation, while vitamin D3 synthesis (cholecalciferol) is uniquely initiated in the skin of vertebrates. During sun exposure, ultraviolet B photons are absorbed by 7-dehydrocholesterol, which is found within the plasma membranes of epidermal and dermal skin layers. This reaction yields an unstable derivative of 7-dehydrocholesterol, named precholecalcitrol, which rapidly rearranges to vitamin D3. Vitamin D binding protein (DBP) is a carrier protein responsible for drawing vitamin D3 from the plasma membrane into the dermal capillaries within the extracellular space.
This application shows an Ascentis Express F5 provided rapid resolution of the isobaric vitamin D metabolites. MS detection provides the necessary secondary resolution.
Verwandter Inhalt
The metabolic role and health implications of the various vitamin D isoforms are of current clinical interest. Therefore, it is important to have an analytical method that will resolve all of the known isoforms with necessary sensitivity and specificity
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.