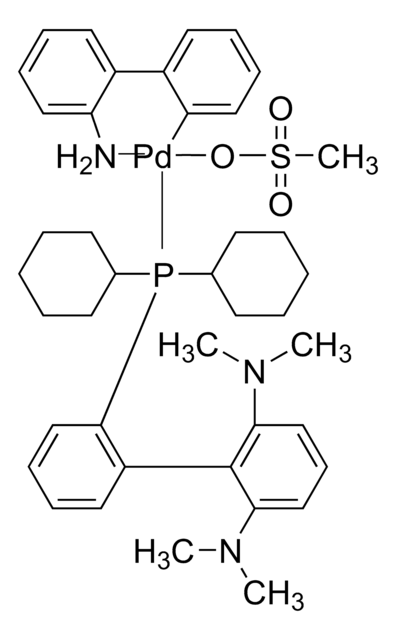
764183
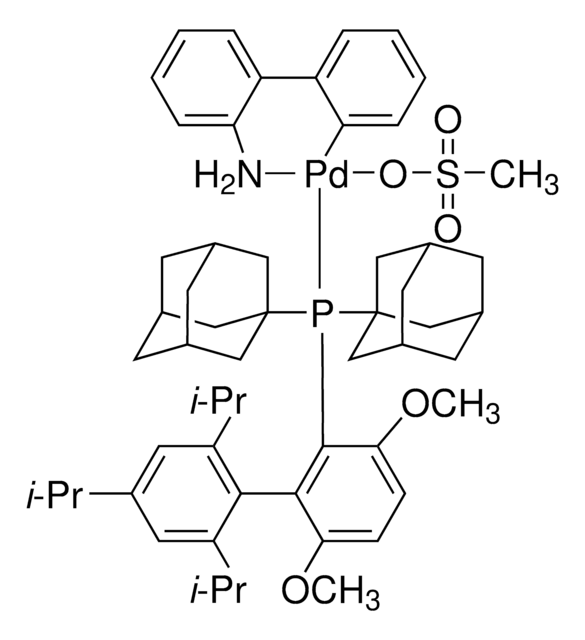
APhos Pd G3
97%
Synonym(e):
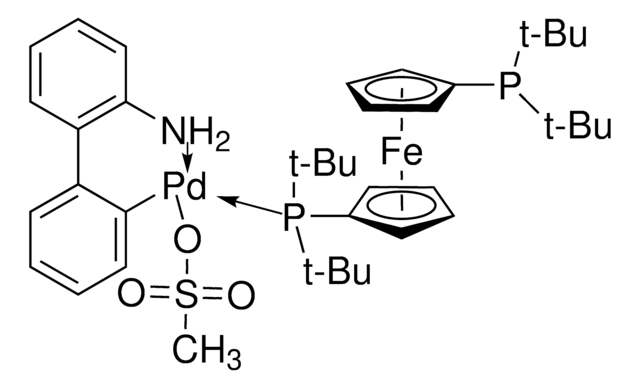
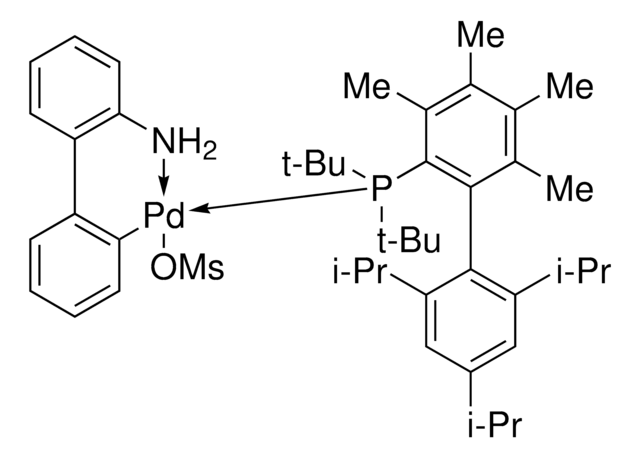
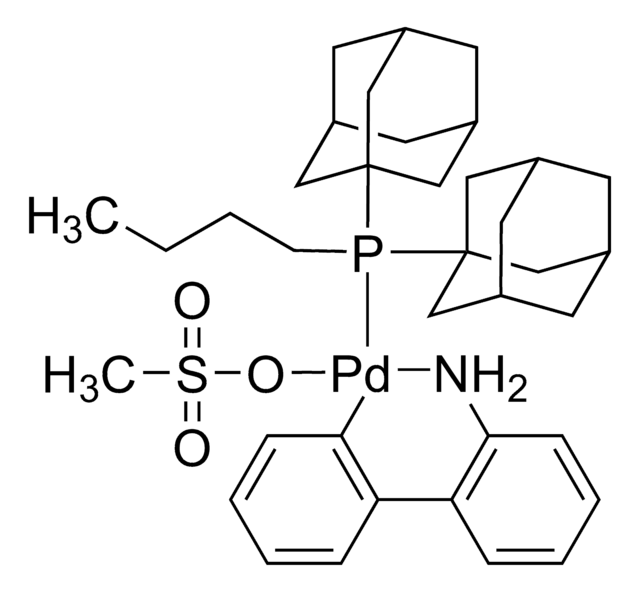
APhos-Pd-G3, Palladium G3-(4-(N,N-Dimethylamino)phenyl)di-tert-butylphosphine, [4-(Di-tert-butylphosphino)-N,N-dimethylaniline-2-(2′-aminobiphenyl)]palladium(II) methanesulfonate
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
97%
Form
solid
Leistungsmerkmale
generation 3
Eignung der Reaktion
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
reaction type: Cross Couplings
Grünere Alternativprodukt-Bewertung
old score: 5
new score: 3
Find out more about DOZN™ Scoring
Grünere Alternativprodukt-Eigenschaften
Waste Prevention
Atom Economy
Less Hazardous Chemical Syntheses
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp (Schmelzpunkt)
192-201 °C (decomposition)
Funktionelle Gruppe
phosphine
Grünere Alternativprodukt-Kategorie
SMILES String
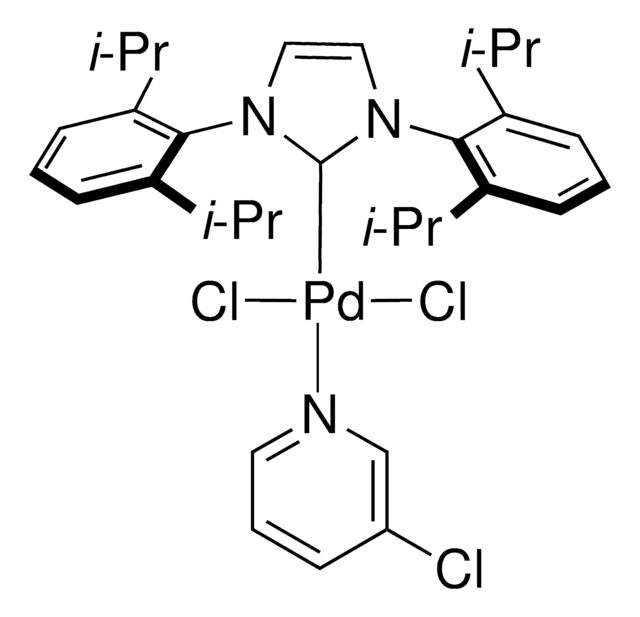
NC1=C(C=CC=C1)C2=C([Pd]OS(C)(=O)=O)C=CC=C2.CN(C)C3=CC=C(C=C3)P(C(C)(C)C)C(C)(C)C
InChI
1S/C16H28NP.C12H10N.CH4O3S.Pd/c1-15(2,3)18(16(4,5)6)14-11-9-13(10-12-14)17(7)8;13-12-9-5-4-8-11(12)10-6-2-1-3-7-10;1-5(2,3)4;/h9-12H,1-8H3;1-6,8-9H,13H2;1H3,(H,2,3,4);/q;;;+1/p-1
InChIKey
SNUBBUQVCDWEAV-UHFFFAOYSA-M
Allgemeine Beschreibung
Anwendung
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
All of the preformed catalysts used in the kit are air and moisture stable complexes in their commercially available form.
Multiple tools have been created to ensure your success with kit set up. Start with the more detailed guide to ensure you are comfortable with all of the steps before using the quick guides on the excel worksheet. Remember that while the technique is new, it is still organic chemistry and so the steps will seem easy once you try just one kit. It is just a new way of approaching something you are already very good at.
Materials Included in your KITALYSIS-24PD-2PK High-Throughput Screening Kit
G3 and G4 Buchwald palladium precatalysts are the newest air, moisture, and thermally stable crossing-coupling complexes used in bond formation for their versatility and high reactivity.
Verwandter Inhalt
The Buchwald group has developed a series of highly active and versatile palladium precatalysts and biarylphosphine ligands used in cross-coupling reactions for the formation of C-C, C–N, C–O, C–F, C–CF3, and C–S bonds. The ligands are electron-rich, and highly tunable to provide catalyst systems with a diverse scope, high stability and reactivity. Furthermore, the new series of precatalysts are air-, moisture and thermally-stable and display good solubility in common organic solvents. The use of precatalysts ensures the efficient generation of the active catalytic species and allows one to accurately adjust the ligand:palladium ratio. The ligands, precatalysts and methodology developed in the Buchwald group are user friendly and have rendered previously difficult cross couplings reactions, much easier to achieve.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.
![[1,1′-Bis(di-tert-butylphosphino)ferrocen]dichlorpalladium(II) 98%](/deepweb/assets/sigmaaldrich/product/structures/192/459/02d1239c-1119-49d9-b392-a04d8f53855c/640/02d1239c-1119-49d9-b392-a04d8f53855c.png)