Alle Fotos(1)
Wichtige Dokumente
366978
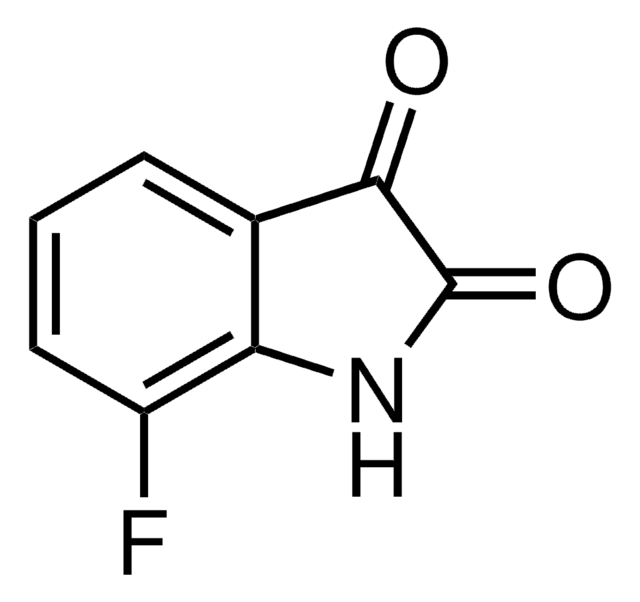
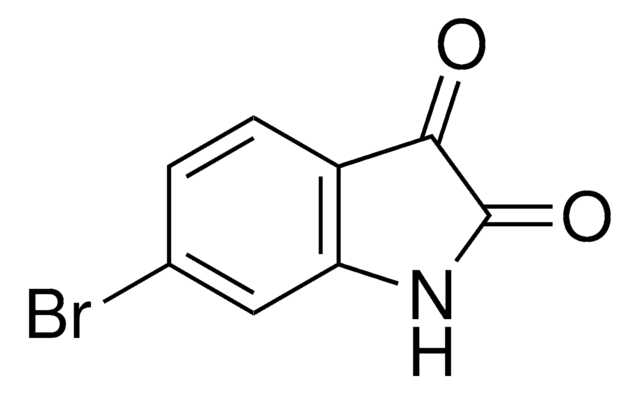
5-Fluorisatin
98%
Synonym(e):
5-Fluoro-2,3-indoledione, NSC 39161
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C8H4FNO2
CAS-Nummer:
Molekulargewicht:
165.12
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
98%
mp (Schmelzpunkt)
224-227 °C (lit.)
Funktionelle Gruppe
fluoro
ketone
SMILES String
Fc1ccc2NC(=O)C(=O)c2c1
InChI
1S/C8H4FNO2/c9-4-1-2-6-5(3-4)7(11)8(12)10-6/h1-3H,(H,10,11,12)
InChIKey
GKODDAXOSGGARJ-UHFFFAOYSA-N
Allgemeine Beschreibung
5-Fluoroisatin has been reported as the precursor of the Sunitinib (Sutent) drug. 5-Fluoroisatin has been approved by the Food and Drugs Administration (FDA) in 2006 for the treatment of renal cell carcinoma (RCC) and gastrointestinal stromal tumor (GIST).
Anwendung
5-Fluoroisatin may be used:
- as reaction-based probe for live-cell detection of peroxynitrite by 19F magnetic resonance spectroscopy
- in non-invasive detection of peroxynitrite (ONOO(-)) formation in living lung epithelial cells stimulated with interferon-γ (IFN-γ)
- in the synthesis of bis-Schiff bases, via condensation with aromatic primary bis-amines in water suspension medium without using any organic solvent or acid catalyst
- in the synthesis of 3-acetonyl-5-fluoro-3-hydroxyoxindole
Reactant for preparation of:
- Spiro[indole-thiazolidinones] as biologically relevan synthesis scaffolds
- Potential antimycobacterial agents
- Inhibitors of c-Met kinase
- Inhibitors of TAK1 kinase
- Herpes simplex virus inhibitors
- IKKβ inhibitors
- Inhibitors of vitiligo disease
- Potential drug candidates with anti-HIV activity and anti-tubercular activity
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
A Novel Preparation of a-Substituted Tryptamines from Isatins.
Franklin CS and White AC.
Journal of the Chemical Society, 2, 1335-1337 (1963)
A A Jarrahpour et al.
Molecules (Basel, Switzerland), 11(1), 59-63 (2007-10-27)
Condensation of aromatic primary bis-amines with isatin (1H-indole-2,3-dione) and 5-flouroisatin occurred cleanly and efficiently in a water suspension medium without using any organic solvent or acid catalyst. The corresponding bis-Schiff bases were obtained in good yields and were easily isolated
Counter-Current chromatography separation of isatin derivatives using the sandmeyer methodology.
Almeida MR, et al.
Journal of the Brazilian Chemical Society, 21(4), 764-769 (2010)
Kevin J Bruemmer et al.
Chemical communications (Cambridge, England), 50(82), 12311-12314 (2014-09-03)
We report a newly discovered oxidative decarbonylation reaction of isatins that is selectively mediated by peroxynitrite (ONOO(-)) to provide anthranilic acid derivatives. We have harnessed this rapid and selective transformation to develop two reaction-based probes, 5-fluoroisatin and 6-fluoroisatin, for the
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.