Alle Fotos(2)
Wichtige Dokumente
160954
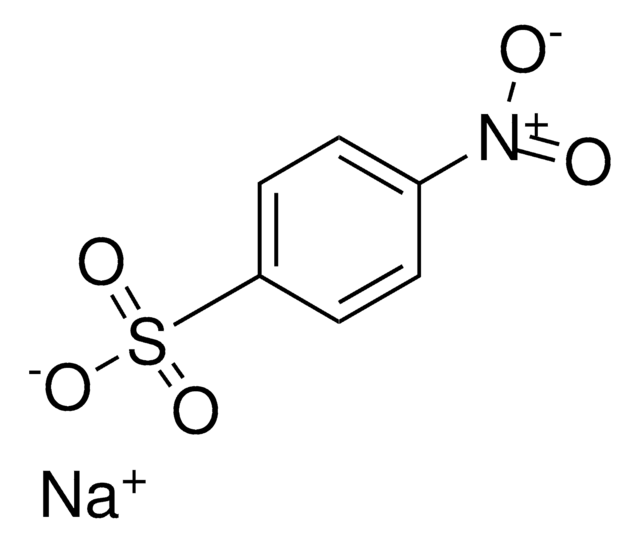
Methyl-4-nitrobenzolsulfonat
99%
Synonym(e):
Methyl nosylate, Methyl p-nitrobenzenesulfonate, Methyl p-nitrotosylate
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(2)
About This Item
Lineare Formel:
O2NC6H4SO3CH3
CAS-Nummer:
Molekulargewicht:
217.20
Beilstein:
2277327
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Assay
99%
Form
solid
mp (Schmelzpunkt)
89-92 °C (lit.)
Löslichkeit
acetone: soluble 5%, clear, faintly yellow to greenish-yellow
Funktionelle Gruppe
nitro
sulfonic acid
SMILES String
COS(=O)(=O)c1ccc(cc1)[N+]([O-])=O
InChI
1S/C7H7NO5S/c1-13-14(11,12)7-4-2-6(3-5-7)8(9)10/h2-5H,1H3
InChIKey
RMNJNEUWTBBZPT-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Reaction between methyl 4-nitrobenzenesulfonate and bromide ions has been studied in mixed single-chain-gemini micellar solutions. Kinetics of SN2 reactions of methyl 4-nitrobenzenesulfonate with ammonia, primary amines, secondary amines, tertiary amines and anionic nucleophiles has been studied.
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
S Ishii et al.
Protein science : a publication of the Protein Society, 7(8), 1802-1810 (1999-03-19)
Aromatic L-amino acid decarboxylase (AADC) catalytic mechanism has been proposed to proceed through two consecutive intermediates (i.e., Michaelis complex and the external aldimine). Limited proteolysis of AADC that preferentially digested at the C-terminal side of Arg334 was slightly retarded in
O Paquatte et al.
Photochemistry and photobiology, 50(6), 817-825 (1989-12-01)
Vibrio harveyi luciferase, an alpha beta dimer, was effectively inactivated by treatment with the methylation agent methyl p-nitrobenzene sulfonate. However, inactivation of luciferase in the presence of excess amounts of this reagent did not follow pseudo-first-order kinetics. After taking the
T Kohzuma et al.
Journal of biochemistry, 106(6), 1054-1058 (1989-12-01)
When Trimeresurus flavoviridis phospholipase A2 was reacted with methyl p-nitrobenzenesulfonate, its activity decreased following first-order kinetics. The pH dependence of the rate constants of inactivation showed that His-48 with an apparent pKa of 6.5 controls the reaction. In the pH
Cysteine modification of metallothionein.
P E Hunziker
Methods in enzymology, 205, 399-400 (1991-01-01)
J P Marcus et al.
Archives of biochemistry and biophysics, 316(1), 413-420 (1995-01-10)
Incubation of L-threonine dehydrogenase from Escherichia coli with methyl p-nitrobenzenesulfonate results in a time- and concentration-dependent loss of enzymatic activity. As the concentration of the methylating agent is increased, the rate of inactivation reaches a limiting value of 0.01 min-1
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.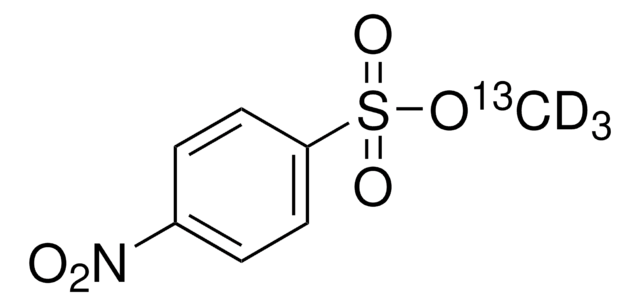


![7-Methyl-1,5,7-triazabicyclo[4.4.0]dec-5-en 98%](/deepweb/assets/sigmaaldrich/product/structures/237/769/028967ef-ca63-4f22-acc9-68f135a43b9a/640/028967ef-ca63-4f22-acc9-68f135a43b9a.png)