1A00900
USP
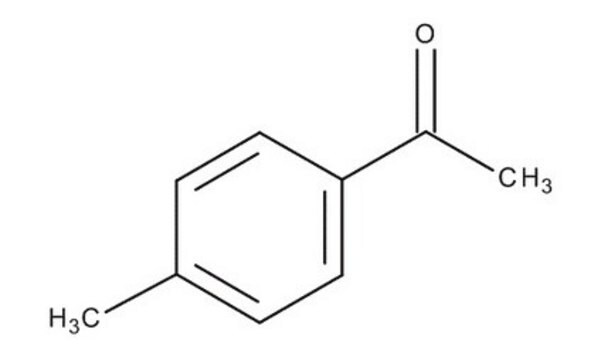
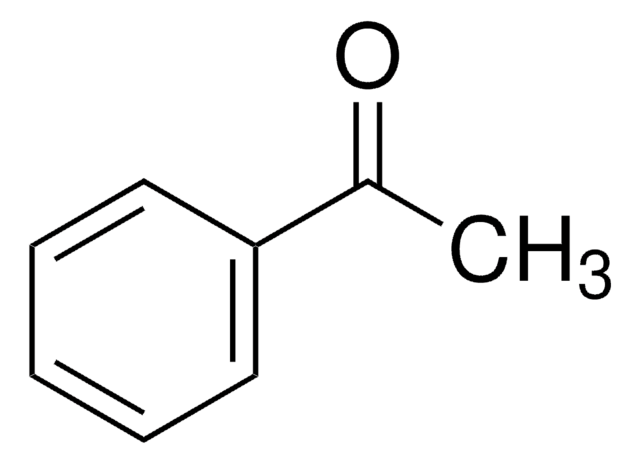
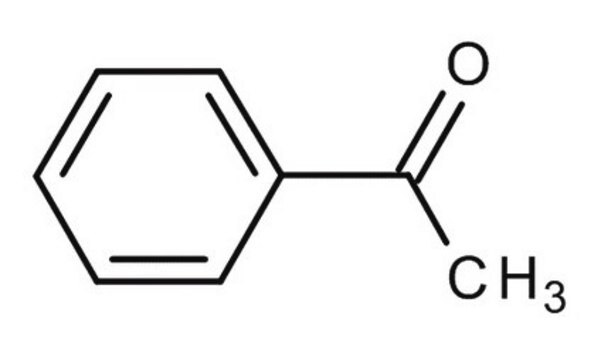
4-Methylacetophenone
Pharmaceutical Analytical Impurity (PAI)
Sinonimo/i:
(1-(4-Methylphenyl)-1-ethanone (4′-Methylacetophenone))
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C9H10O
Numero CAS:
Peso molecolare:
134.18
Codice UNSPSC:
41116100
NACRES:
NA.24
Prodotti consigliati
Grado
pharmaceutical analytical impurity (PAI)
agenzia
USP
Produttore/marchio commerciale
USP
applicazioni
pharmaceutical
Formato
neat
Temperatura di conservazione
2-8°C
Descrizione generale
4-Methylacetophenone is a USP Pharmaceutical Analytical Impurity (PAI).
USP PAI are a product line of impurities suitable for research and analytical purposes, which help to ensure the quality and safety of medicines.
Associated Drug Substance: Celecoxib
Therapeutic Area: Analgesics
For more information about this PAI, visit here.
USP PAI are a product line of impurities suitable for research and analytical purposes, which help to ensure the quality and safety of medicines.
Associated Drug Substance: Celecoxib
Therapeutic Area: Analgesics
For more information about this PAI, visit here.
Applicazioni
4-Methylacetophenone (USP PAI) is intended for use in analytical testing to detect, identify, and measure pharmaceutical impurities.
Caratteristiche e vantaggi
USP PAI advance your early analytical R&D and process development. PAI can be used in the following applications:
1. Conduct analytical tests during early formulation feasibility studies.
2. Determine degradation impurities produced during stress studies.
3. Develop, validate, and transfer analytical methods.
4. Perform spiking studies during process R&D to demonstrate depletion upon recrystallization.
5. Record retention times and/or spectra
6. Determine relative response factors.
7. Identify unknown impurities that formed during ICH stability conditions.
8. Identify impurities that are present in the Reference Listed Drug
9. Test for and profile impurities not listed in drug substance and drug product monographs.
1. Conduct analytical tests during early formulation feasibility studies.
2. Determine degradation impurities produced during stress studies.
3. Develop, validate, and transfer analytical methods.
4. Perform spiking studies during process R&D to demonstrate depletion upon recrystallization.
5. Record retention times and/or spectra
6. Determine relative response factors.
7. Identify unknown impurities that formed during ICH stability conditions.
8. Identify impurities that are present in the Reference Listed Drug
9. Test for and profile impurities not listed in drug substance and drug product monographs.
Risultati analitici
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
Altre note
Sales restrictions may apply.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Skin Irrit. 2
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 1
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Lot/Batch Number
Ci dispiace, ma al momento non ci sono COA disponibili online per questo prodotto.
Se ti serve aiuto, non esitare a contattarci Servizio Clienti
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.