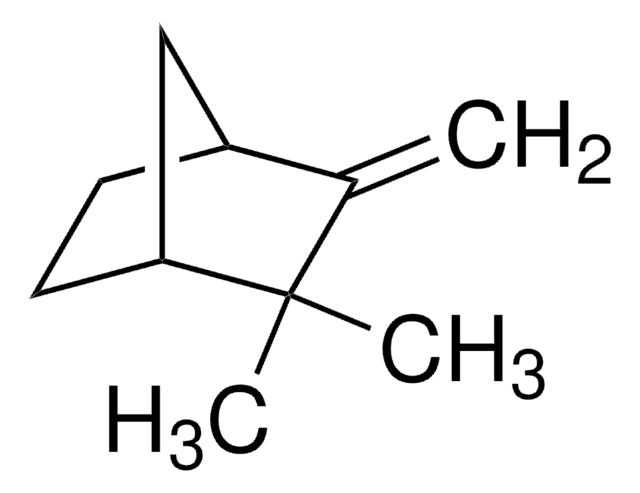
442505
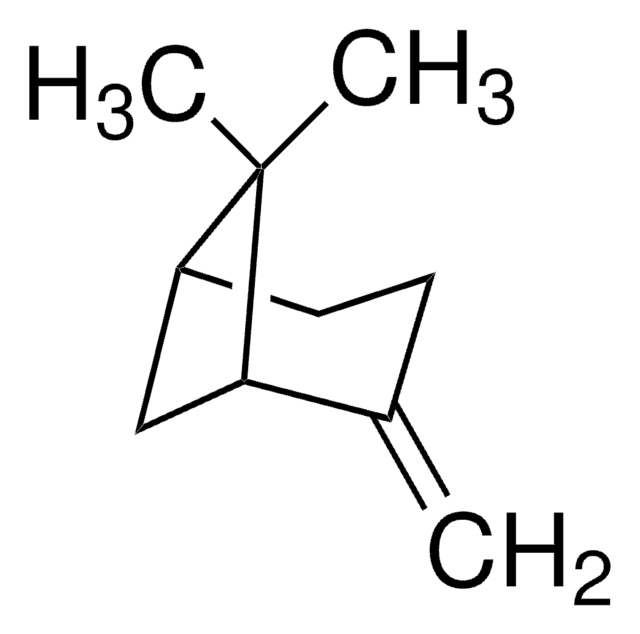
Camphene
analytical standard
Sinonimo/i:
DL-Camphene
About This Item
Prodotti consigliati
Grado
analytical standard
CdA
current certificate can be downloaded
Confezionamento
ampule of 1000 mg
tecniche
HPLC: suitable
gas chromatography (GC): suitable
P. ebollizione
159-160 °C (lit.)
Punto di fusione
48-52 °C (lit.)
Densità
0.85 g/mL at 25 °C (lit.)
applicazioni
cleaning products
cosmetics
environmental
flavors and fragrances
food and beverages
personal care
Formato
neat
Temperatura di conservazione
2-30°C
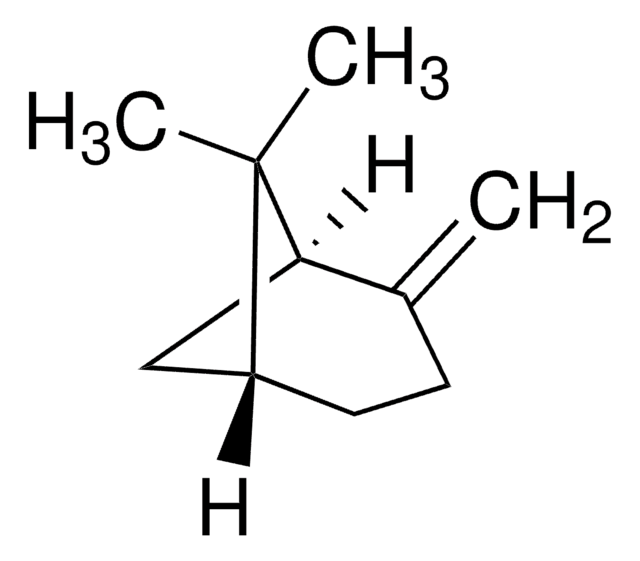
Stringa SMILE
[H][C@]12CC[C@]([H])(C1)C(C)(C)C2=C
InChI
1S/C10H16/c1-7-8-4-5-9(6-8)10(7,2)3/h8-9H,1,4-6H2,2-3H3/t8-,9+/m0/s1
CRPUJAZIXJMDBK-DTWKUNHWSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Flam. Sol. 1
Codice della classe di stoccaggio
4.1B - Flammable solid hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
78.8 °F - DIN 51755 Part 1
Punto d’infiammabilità (°C)
26 °C - DIN 51755 Part 1
Dispositivi di protezione individuale
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Protocolli
-Pinocarveol; Menthol; (+)-Terpinen-4-ol; α-Terpineol; (±)-α-Terpinyl acetate, predominantly α-isomer; Germacrene D
Cymene; 4,5,6,7-Tetrahydro-3,6-dimethylbenzofuran; Linalool; Menthol; Menthone; Menthyl acetate; Germacrene D; Bicyclogermacrene; Thymol
-α-Bergamotene; β-Bisabolene; α-Terpineol; Neryl acetate; Geranyl acetate; Neral; Geranial
-3,7-Dimethyl-2,6-octadien-1-ol; Neral; Geraniol; Geranial; Undecanal; Citronellyl acetate; Neryl acetate; 3,7-Dimethyl-2,6-octadienyl acetate; 1-Tetradecene; Tetradecane; α-Bisabolol
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.