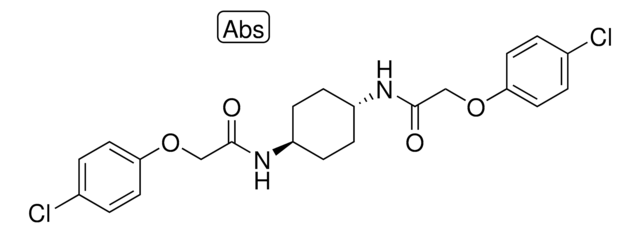
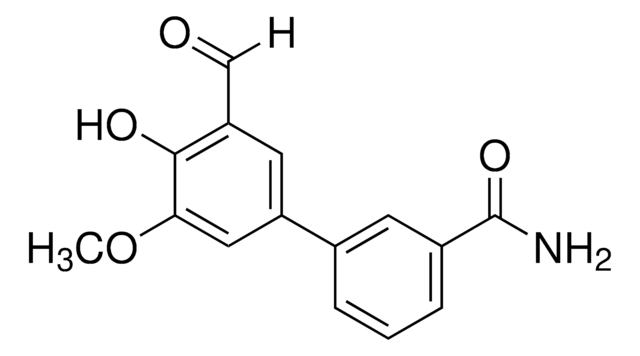
SML0409
STF-083010
≥98% (HPLC)
Sinonimo/i:
N-[(2-Hydroxy-1-naphthalenyl)methylene]-2-thiophenesulfonamide
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
≥98% (HPLC)
Stato
powder
Colore
light yellow to yellow-green
Solubilità
DMSO: 5 mg/mL (clear solution)
Temperatura di conservazione
−20°C
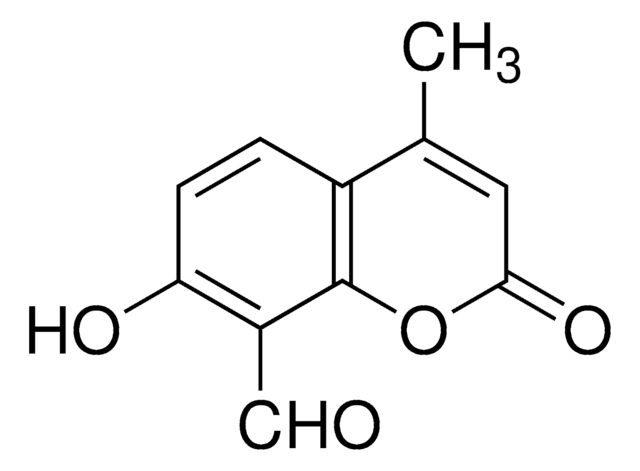
Stringa SMILE
OC1=C(/C=N/S(C2=CC=CS2)(=O)=O)C3=C(C=CC=C3)C=C1
InChI
1S/C15H11NO3S2/c17-14-8-7-11-4-1-2-5-12(11)13(14)10-16-21(18,19)15-6-3-9-20-15/h1-10,17H/b16-10+
TVIVJHZHPKNDAQ-MHWRWJLKSA-N
Applicazioni
- in a study to investigate the potential anti-lipotoxic effect of nicotinamide and to elucidate underlying mechanism(s)
- as IRE1a inhibitor to study its effect on NOS 2 expression and investigate the underlying mechanisms in proinflammatory gene expression in astrocytes
- to block endogenous XBP1 cleavage for one hour prior to palmitate exposure in order to examine whether inositol?requiring enzyme 1α (IRE1α ) activation is implicated in palmitate cytotoxicity
Azioni biochim/fisiol
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Non trovi la versione di tuo interesse?
Se hai bisogno di una versione specifica, puoi cercare il certificato tramite il numero di lotto.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.

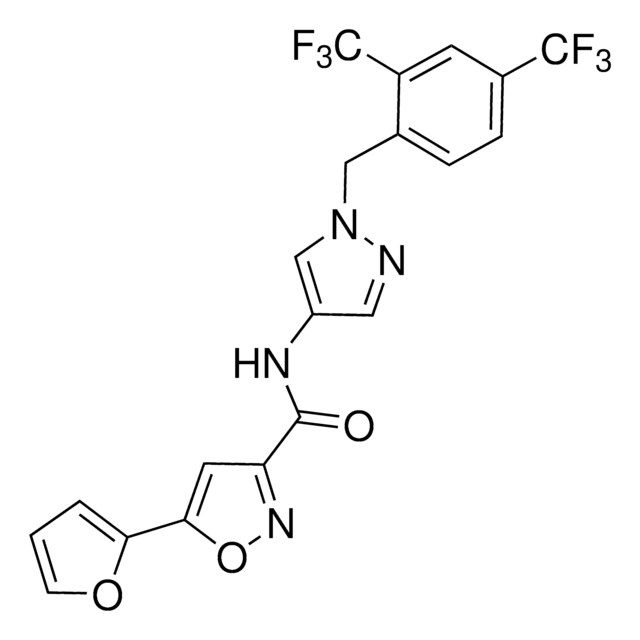



![PERK Inhibitor I, GSK2606414 GSK2606414 is a cell-permeable, highly potent inhibitor of EIF2AK3/PERK (IC₅₀ = 0.4 nM; [ATP] = 5 µM). Targets PERK in its inactive DFG conformation at the ATP-binding region.](/deepweb/assets/sigmaaldrich/product/structures/180/559/efa716dc-d5fe-4339-a6f0-0103084fc04a/640/efa716dc-d5fe-4339-a6f0-0103084fc04a.png)