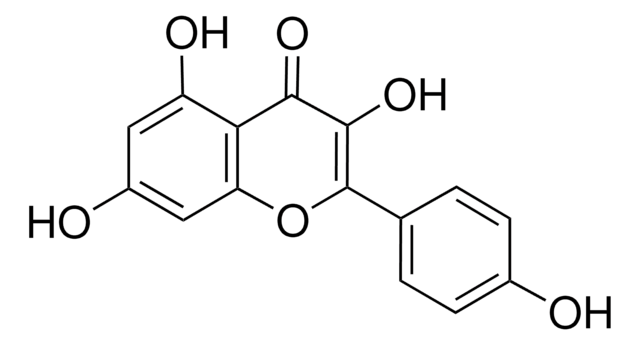
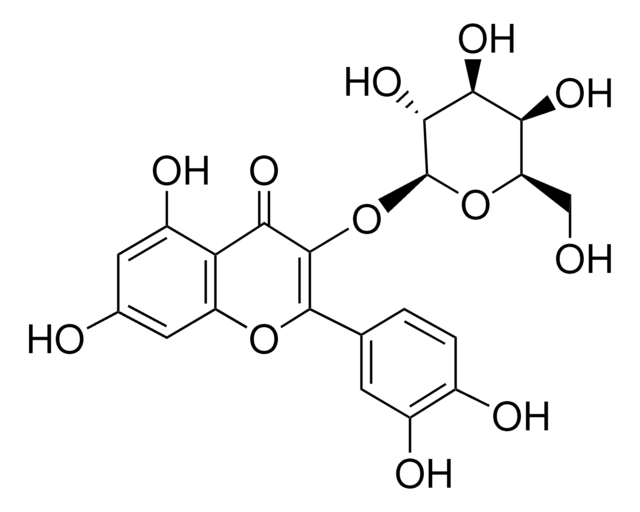
R5143
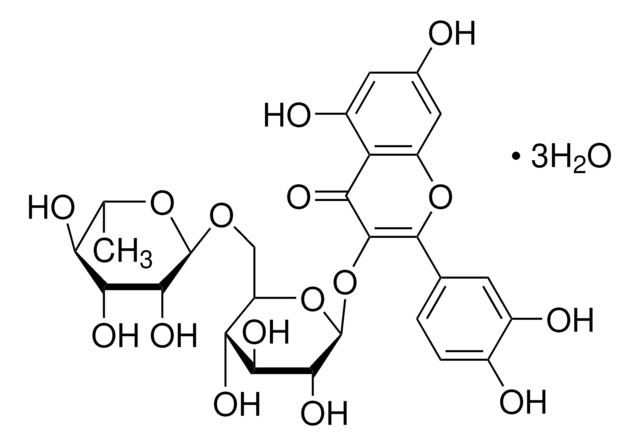
Rutin hydrate
≥94% (HPLC), powder
Sinonimo/i:
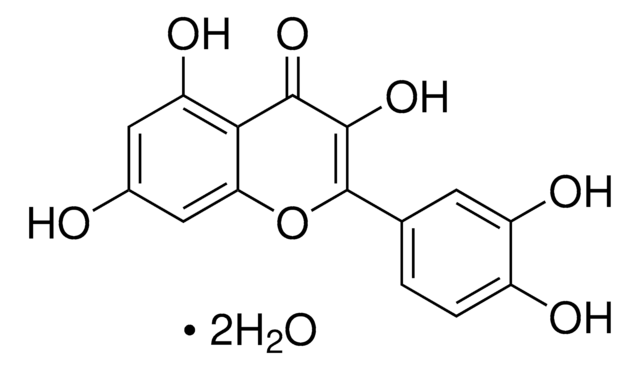
Quercetin-3-rutinoside, Vitamin P
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
≥94% (HPLC)
Forma fisica
powder
Colore
yellow to green
Punto di fusione
195 °C (dec.) (lit.)
Solubilità
pyridine: 50 mg/mL
DMSO: soluble
aqueous base: soluble
applicazioni
metabolomics
vitamins, nutraceuticals, and natural products
Stringa SMILE
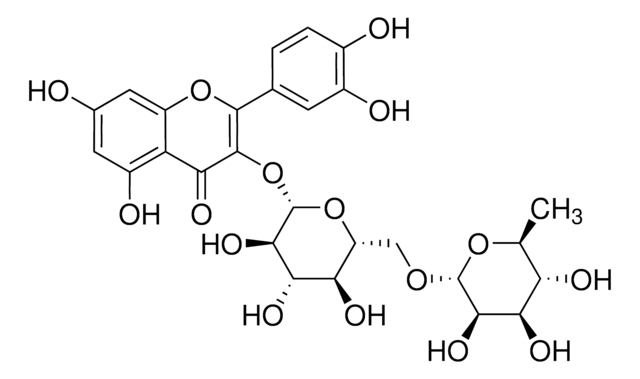
[H]O[H].O[C@H]1[C@H](OC[C@H]([C@H]2O)O[C@@H](OC(C3=O)=C(C4=CC=C(O)C(O)=C4)OC5=C3C(O)=CC(O)=C5)[C@H](O)[C@H]2O)O[C@H]([C@@H]([C@H]1O)O)C
InChI
1S/C27H30O16.H2O/c1-8-17(32)20(35)22(37)26(40-8)39-7-15-18(33)21(36)23(38)27(42-15)43-25-19(34)16-13(31)5-10(28)6-14(16)41-24(25)9-2-3-11(29)12(30)4-9;/h2-6,8,15,17-18,20-23,26-33,35-38H,7H2,1H3;1H2/t8-,15+,17-,18+,20+,21-,22+,23+,26+,27-;/m0./s1
PGHSKTKIQIBATG-ZAAWVBGYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
- as an inhibitory compound against Skeletonema costatum
- to analyze the phenolic component and antioxidant activity of nettle
- to assess the flavonoid content in Propolis
Azioni biochim/fisiol
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Protocolli
This application illustrate how Rutin can be determined in skin cream samples using a monolithic Chromolith® HighResolution RP-18 endcapped column with UV detection.
HPLC Analysis of Polyphenols in Nero d'Avola Red Wine on Discovery® HS C18 (UV 280 nm)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.