17793
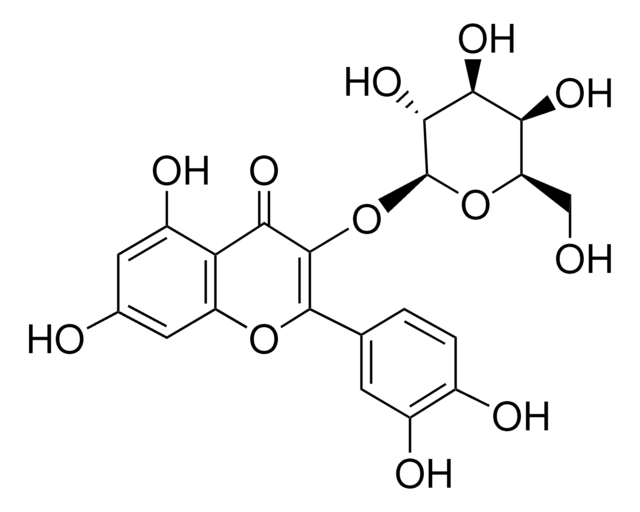
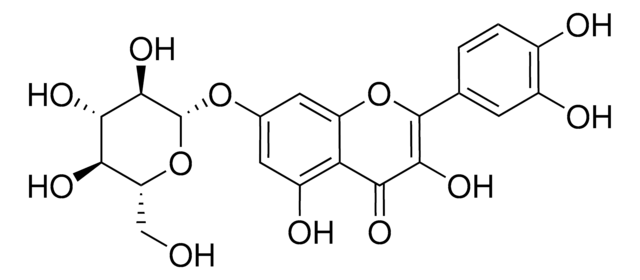
Quercetin 3-β-D-glucoside
≥90% (HPLC)
Sinonimo/i:
3,3′,4′,5,7-Pentahydroxyflavone 3-β-glucoside, Isoquercitrin
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
≥90% (HPLC)
applicazioni
metabolomics
vitamins, nutraceuticals, and natural products
Temperatura di conservazione
−20°C
Stringa SMILE
OC[C@H]1O[C@@H](OC2=C(Oc3cc(O)cc(O)c3C2=O)c4ccc(O)c(O)c4)[C@H](O)[C@@H](O)[C@@H]1O
InChI
1S/C21H20O12/c22-6-13-15(27)17(29)18(30)21(32-13)33-20-16(28)14-11(26)4-8(23)5-12(14)31-19(20)7-1-2-9(24)10(25)3-7/h1-5,13,15,17-18,21-27,29-30H,6H2/t13-,15-,17+,18-,21+/m1/s1
OVSQVDMCBVZWGM-QSOFNFLRSA-N
Informazioni sul gene
mouse ... Hexa(15211)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
- as a dietary flavonoid supplement to check its binding capacity with human small ubiquitin-related modifier 1 (SUMO1) protein using surface plasmon resonance (SPR)
- as an inhibitor for Escherichia coli adenosine triphosphate (ATP) synthase
- as an anti-aggregation agent to test its activity against β-amyloid, green fluorescent protein (GFP), and chymotrypsinogen proteins
Azioni biochim/fisiol
Confezionamento
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Protocolli
HPLC Analysis of Polyphenols in Nero d'Avola Red Wine on Discovery® HS C18 (UV 280 nm)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.