M9070
Matrix Metalloproteinase-2
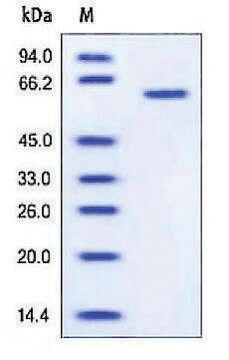
>90% (SDS-PAGE), recombinant, expressed in NSO cells, lyophilized powder
Sinonimo/i:
72 Kd Gelatinase, 72 Kd Type IV Collagenase, Gelatinase A, MMP-2
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Prodotti consigliati
Ricombinante
expressed in NSO cells
Livello qualitativo
Saggio
>90% (SDS-PAGE)
Stato
lyophilized powder
PM
apparent mol wt ~69 kDa
N° accesso UniProt
Temperatura di conservazione
−20°C
Informazioni sul gene
human ... MMP2(4313)
Descrizione generale
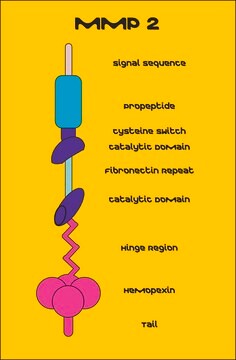
Matrix metalloproteinase-2 (MMP-2) also known as gelatinase or type IV collagenase is a 72kDa protein. MMP-2 is a member of matrix metalloproteinase (MMP) family of enzymes. Basic structure of MMP2 contains signal peptide domain that targets the enzyme for secretion, the pro-peptide domain, which is removed when the enzyme is activated and the catalytic site containing gelatin-binding domain.
Specificità
MMP-2 specifically cleaves type IV collagen, a major structural component of basement membranes.
Applicazioni
Matrix metalloproteinase-2 (MMP2) human has been used:
- As a standard in zymography to measure gelatinolytic activity of MMP2.
- In enzymatic method for dissociation of brain tissue to single cells.
Azioni biochim/fisiol
MMP-2 degrades general matrix components and may have a role in processes such as host defense, cell proliferation, and protein turnover as well as tissue remodeling.
Matrix metalloproteinase-2 (MMP-2) cleaves gelatin, type IV, V, VII, X, and XI collagens, fibronectin, elastin, laminin, proteoglycans, and a range of non extracellular matrix (ECM ) components. MMP-2 cleaves native type I collagen to N-terminal ¾ and C-terminal ¼ fragments identical to those generated by interstitial collagenases. MMP2 and MMP9 play an essential role in matrix degradation and they are implicated in the maintenance of neovascularization. MMP-2 activity is associated with human ovarian follicular development. MMP2 expression is elevated in human myocardial infarction and dilated cardiomyopathy. Wound fluid from chronic leg ulcers show elevated expression of MMP2. Overexpression of active MMP2 affects wound healing in chronic leg ulcers. MMP2 activity is inhibited by tissue inhibitor of metalloproteinase-2 (TIMP-2). Human follicular fluid MMP-2 level acts as a potential biomarker of oocyte maturation in in vitro fertilization and intracytoplasmic sperm injection (ICSI) cycles.
Stato fisico
Lyophilized from a 0.2 μm filtered solution of 50 mM Tris, 5 mM CaCl2, 150 mM NaCl, and 1 μM ZnCl2, pH 7.5.
Ricostituzione
It is recommended that 0.1 mL of buffer (100 mM Tris, 10 mM calcium chloride, 150 mM sodium chloride, and 0.05% Brij® L23, pH 8.0) be used to give a stock solution of the enzyme at 100 μg/mL.
Risultati analitici
The biological activity is measured by its ability to cleave a fluorogenic peptide substrate.
Note legali
Brij is a registered trademark of Croda International PLC
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Immunohistochemical expression of MMP-14 and MMP-2, and MMP-2 activity during human ovarian follicular development.
Vos MC
Reproductive Biology and Endocrinology, 12:12 (2014)
Matrix metalloproteinase 2 level in human follicular fluid is a reliable marker of human oocyte maturation in in vitro fertilization and intracytoplasmic sperm injection cycles.
Yang WJ
Reproductive Biology and Endocrinology, 13:102 (2015)
A non-aggressive, highly efficient, enzymatic method for dissociation of human brain-tumors and brain-tissues to viable single-cells.
Volovitz I
BMC Neuroscience, 17(1):30 (2016)
Takashi Temma et al.
PloS one, 9(7), e102180-e102180 (2014-07-11)
Since matrix metalloproteinase-2 (MMP-2) is an important marker of tumor malignancy, we developed an original drug design strategy, MMP-2 activity dependent anchoring probes (MDAP), for use in MMP-2 activity imaging, and evaluated the usefulness of this probe in in vitro
Matrix metalloproteinases and myocardial infarction
Phatharajaree W
The Canadian Journal of Cardiology, 23(9), 727-733 (2007)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.