04685
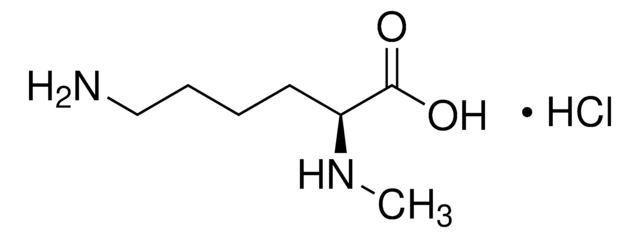
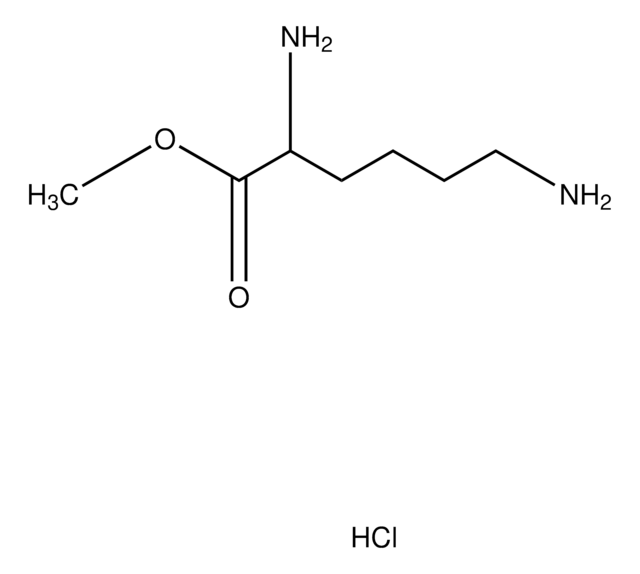
Nε-Methyl-L-lysine hydrochloride
≥98.0% (TLC)
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C7H16N2O2 · HCl
Numero CAS:
Peso molecolare:
196.68
Numero CE:
Numero MDL:
Codice UNSPSC:
12352202
ID PubChem:
NACRES:
NA.32
Saggio:
≥98.0% (TLC)
Prodotti consigliati
Livello qualitativo
Saggio
≥98.0% (TLC)
Attività ottica
[α]/D 20.5±1.5°, c = 0.1 in 1 M HCl
Temperatura di conservazione
2-8°C
Stringa SMILE
Cl.CNCCCC[C@H](N)C(O)=O
InChI
1S/C7H16N2O2.ClH/c1-9-5-3-2-4-6(8)7(10)11;/h6,9H,2-5,8H2,1H3,(H,10,11);1H/t6-;/m0./s1
AQELUQTVJOFFBN-RGMNGODLSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Azioni biochim/fisiol
N ε-methyl-L-lysine was identified as a lysine analog with inhibitory effects on the growth and sporulation of Penicillium chrysogenum and benzyl-penicillin formation by mycelia.
Confezionamento
Bottomless glass bottle. Contents are inside inserted fused cone.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
F Zappacosta et al.
European journal of biochemistry, 222(3), 761-767 (1994-06-15)
Advanced mass spectrometric procedures have been extensively used to provide an accurate structural characterization of aspartate aminotransferase from Sulfolobus solfataricus. The amino acid sequence of this enzyme had previously been deduced from the DNA sequence. The accurate molecular mass of
H Kalász et al.
Journal of chromatographic science, 43(4), 165-168 (2005-06-25)
Administration of (14)C-labelled L-deprenyl to rats results in the urinary elimination of a 14C-labelled compound. The 9-fluorenylmethoxycarbonyl chloride-reacted urine sample is fractionated by high-performance liquid chromatography (HPLC) on an octadecyl silica stationary phase. N(epsilon)-Monomethyl-lysine is identified in the fraction containing
M Moracci et al.
Enzyme and microbial technology, 17(11), 992-997 (1995-11-01)
The gene coding for the beta-glycosidase from the archaeon Sulfolobus solfataricus has been overexpressed in Escherichia coli. The enzyme was purified to homogeneity with a rapid purification procedure employing a thermal precipitation as a crucial step. The final yield was
H Kalász et al.
Journal of chromatography. A, 1079(1-2), 208-212 (2005-07-26)
Nepsilon-Monomethyllysine was identified in the serum, urine, brain, and liver samples of rats treated per os with L-deprenyl. The identification procedure included reaction with Fmoc chloride, clean-up, and analysis using HPLC-UV-MS. Oral administration of (-)-N-14C-methyl-N-propynyl(2-phenyl-1-methyl)ethylammonium hydrochloride L-deprenyl) to rats resulted
M Kushiro et al.
Nephron, 79(4), 458-468 (1998-08-05)
Increases in extracellular matrix (ECM) and changes in its components have been documented in the glomeruli of diabetic nephropathy. Advanced glycation end products formed by glycoxidation have been shown to induce the synthesis of ECM components and transforming growth factor
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.