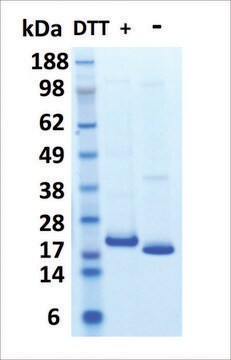
S0947000
Somatropin
European Pharmacopoeia (EP) Reference Standard
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Prodotti consigliati
Grado
pharmaceutical primary standard
Famiglia di API
somatropin
Produttore/marchio commerciale
EDQM
applicazioni
pharmaceutical (small molecule)
Formato
neat
Temperatura di conservazione
−20°C
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
Applicazioni
Somatropin EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
Confezionamento
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
Altre note
Sales restrictions may apply.
Prodotti correlati
N° Catalogo
Descrizione
Determinazione del prezzo
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Lot/Batch Number
Ci dispiace, ma al momento non ci sono COA disponibili online per questo prodotto.
Se ti serve aiuto, non esitare a contattarci Servizio Clienti
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
E Elowe-Gruau et al.
Revue medicale suisse, 10(418), 424-424 (2014-03-20)
Children born premature and/or small for gestational age (SGA) are at risk of growth and metabolic abnormalities. Catch-up growth occurs usually before the age of 2. In the absence of sufficient catch up growth, growth hormone (GH) treatment should be
Małgorzata Partyka et al.
Polski merkuriusz lekarski : organ Polskiego Towarzystwa Lekarskiego, 36(211), 63-67 (2014-03-22)
Growth hormone (GH) is a polypeptide hormone produced by the cells of pituitary. Production of growth hormone is carried out in a pulsating manner, and the frequency and intensity of the pulses is dependent on age and gender. Growth hormone
A Thankamony et al.
The Journal of clinical endocrinology and metabolism, 99(2), 639-647 (2014-01-16)
Data on the metabolic effects of GH derived from studies using GH suppression by pharmacological agents may not reflect selective actions. The purpose of this study was to evaluate the effects of GH antagonism on glucose and lipid metabolism using
Mark Sherlock et al.
The Journal of clinical endocrinology and metabolism, 99(2), 478-485 (2013-11-19)
Acromegaly is associated with reduced life expectancy, which has been reported to be normalized if treatment is successful in controlling GH/IGF-I levels. Most previous studies have invariably used the last available GH/IGF-I, which may be biased as it only assesses
J R Delanghe et al.
Acta clinica Belgica, 69(1), 25-29 (2014-03-19)
The recent Armstrong case, where more than 250 negative doping tests are confronted with the athlete's confession of erythropoietin use, blood doping, steroid, and growth hormone abuse, illustrates the limitations of current laboratory tests in detecting doping in sport. Despite
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.