PHR1669
Lidocaine Related Compound A
Pharmaceutical Secondary Standard; Certified Reference Material
Sinonimo/i:
2,6-Dimethylaniline, Lidocaine Impurity A; 2,6 DMA, 2,6-Xylidine, 2-Amino-1,3-dimethylbenzene, 2-Amino-m-xylene
About This Item
Prodotti consigliati
Grado
certified reference material
pharmaceutical secondary standard
Livello qualitativo
agenzia
traceable to Ph. Eur. Y0001575
Tensione di vapore
<0.01 mmHg ( 20 °C)
Famiglia di API
lidocaine
CdA
current certificate can be downloaded
Confezionamento
pkg of 100 mg
tecniche
HPLC: suitable
gas chromatography (GC): suitable
Indice di rifrazione
n20/D 1.560 (lit.)
P. eboll.
214 °C/739 mmHg (lit.)
Punto di fusione
10-12 °C (lit.)
Densità
0.984 g/mL at 25 °C (lit.)
applicazioni
pharmaceutical (small molecule)
Formato
neat
Temperatura di conservazione
2-30°C
Stringa SMILE
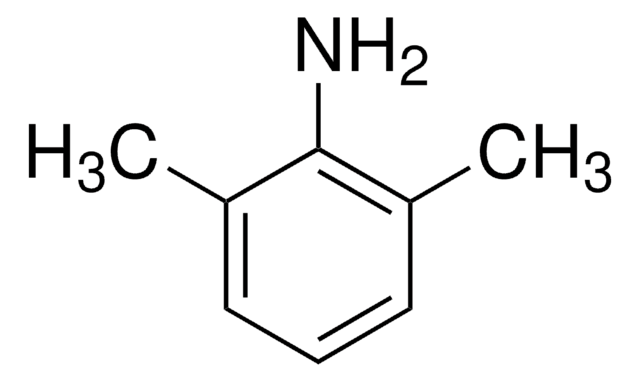
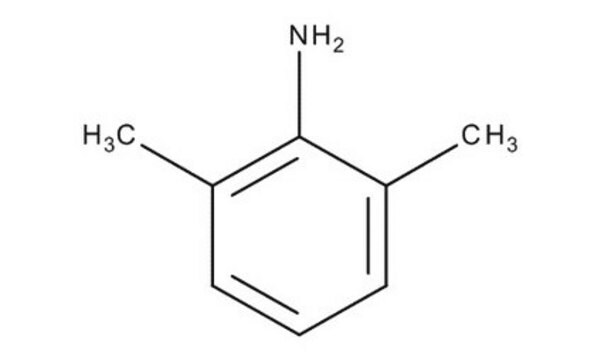
Cc1cccc(C)c1N
InChI
1S/C8H11N/c1-6-4-3-5-7(2)8(6)9/h3-5H,9H2,1-2H3
UFFBMTHBGFGIHF-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
The standard is a certified reference material (CRM) qualified with instruments validated according to good manufacturing practices (GMP) using pharmacopeia monograph methods. It is supplied with a comprehensive certificate containing information on traceability assay results, certified purity, homogeneity tests, uncertainty statement, and stability assessment.
Lidocaine Related Compound A is a primary aromatic amine and a major metabolite of the anesthetic lidocaine. It is used as a starting material in the manufacturing of various anesthetics like lidocaine, bupivacaine, mepivacaine, etidocaine, ropivacaine, pyrrocaine, and xylazine.
Applicazioni
This pharmaceutical secondary standard can also be used as follows:
- Development of an impurity selective reverse phase-high performance liquid chromatography (RP-HPLC) method to determine dexpanthenol, lidocaine hydrochloride, mepyramine maleate, and their related substances in topical dosage forms
- Testing a selective high-performance liquid chromatography-diode array detection (HPLC-DAD) method, developed for the simultaneous analysis of miconazole nitrate and lidocaine hydrochloride in their combined oral gel dosage form, for its stability-indicating properties
- Evaluation of a high-performance liquid chromatography-diode array detection (HPLC-DAD) procedure― for its stability indicating properties, developed to determine nitrofurazone and lidocaine hydrochloride in their combined dosage form
- Separation of 2,6-Dimethylaniline, its isomeric impurities, and other related impurities by isocratic and reverse-phase ultra-performance liquid chromatographic (UPLC) method
- analyze a binary mixture of lidocaine hydrochloride and cetylpyridinium chloride in presence of lidocaine impurity A by spectrophotometric methods
- determine lidocaine hydrochloride-related substance by analytical methods in pharmaceutical dosage forms
Risultati analitici
Nota a piè di pagina
Prodotti consigliati
Prodotti correlati
Avvertenze
Warning
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 2 - Carc. 2 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
195.8 °F - closed cup
Punto d’infiammabilità (°C)
91 °C - closed cup
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Protocolli
GC Analysis of Anilines on Equity®-5
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.