PHR1363
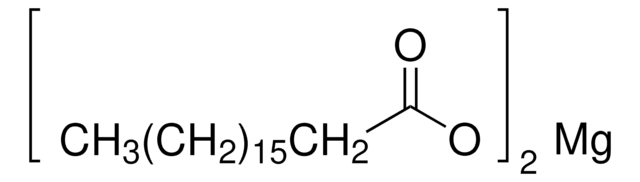
Magnesium Stearate
Pharmaceutical Secondary Standard; Certified Reference Material
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Prodotti consigliati
Grado
certified reference material
pharmaceutical secondary standard
Livello qualitativo
Famiglia di API
magnesium stearate
Confezionamento
ampule of 2.5 g
applicazioni
pharmaceutical (small molecule)
Formato
neat
Temperatura di conservazione
2-30°C
Descrizione generale
Magnesium stearate is commonly utilized as a lubricant in pharmaceutical formulations to avoid the excipient blend from the formulation contents from sticking to the manufacturing equipment.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Applicazioni
Magnesium stearate may be used as an excipient for the manufacturing of atorvastatin drug and poloxamers. The determination of the analyte in pharmaceutical formulations can be carried out by chromatography and colorimetric methods.
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
Risultati analitici
These secondary standards offer multi-traceability to the USP, EP (PhEur) and BP primary standards, where they are available.
Altre note
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
Nota a piè di pagina
To see an example of a Certificate of Analysis for this material enter LRAA1467 in the slot below. This is an example certificate only and may not be the lot that you receive.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
nwg
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Choose from one of the most recent versions:
Certificati d'analisi (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Analysis of magnesium from magnesium stearate in pharmaceutical tablet formulations using hydrophilic interaction liquid chromatography with nano quantity analyte detection
Risley DS, et al.
Journal of Pharmaceutical and Biomedical Analysis, 78, 112-117 (2013)
Liquid chromatographic determination of atorvastatin in bulk drug, tablets, and human plasma
Altuntas TG and Erk N
Journal of Liquid Chromatography and Related Technologies, 27(1), 83-93 (2004)
Quantitation of poloxamers in pharmaceutical formulations using size exclusion chromatography and colorimetric methods
Mao Y, et al.
Journal of Pharmaceutical and Biomedical Analysis, 35(5), 1127-1142 (2004)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.