C112402
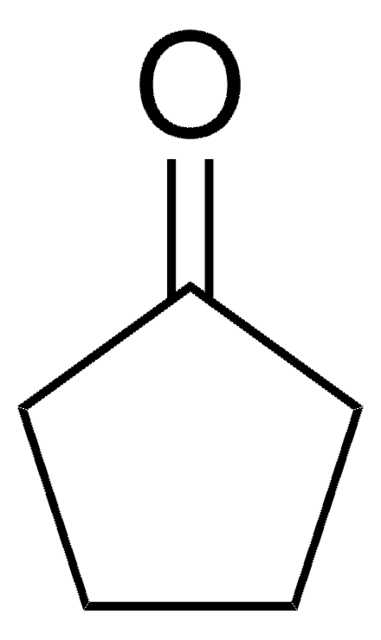
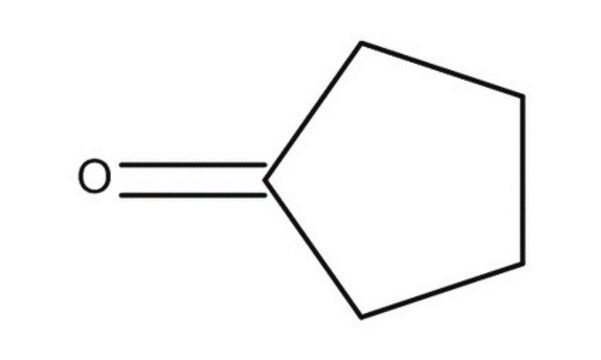
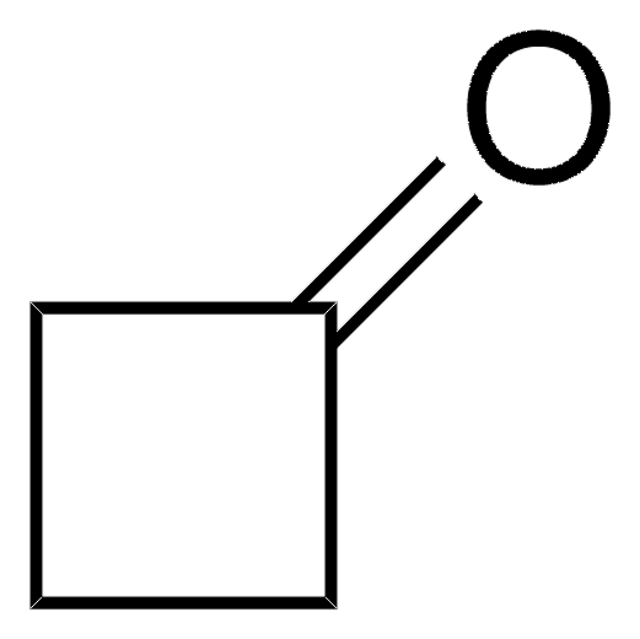
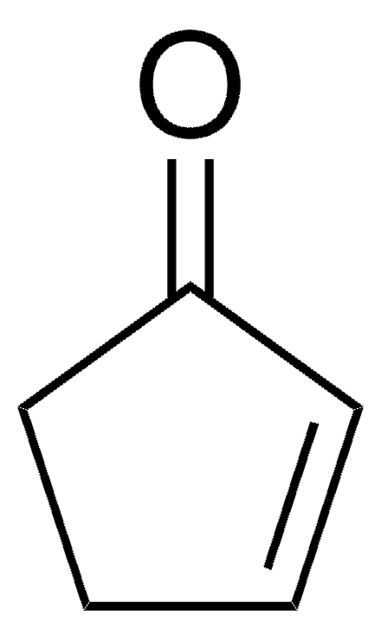
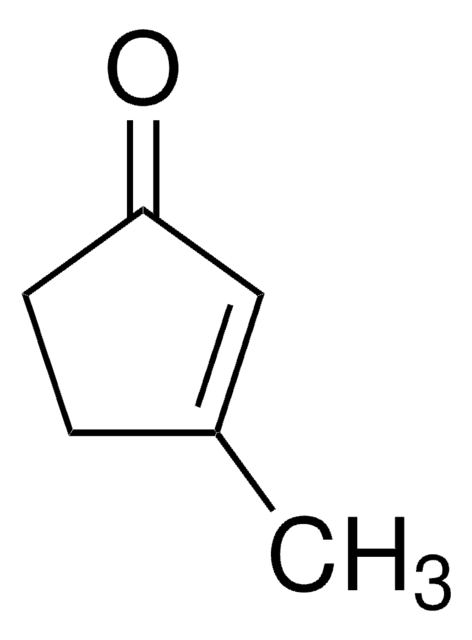
Cyclopentanone
ReagentPlus®, ≥99%
Sinonimo/i:
Adipic ketone
About This Item
Prodotti consigliati
Grado
reagent
Livello qualitativo
Nome Commerciale
ReagentPlus®
Saggio
≥99%
Stato
liquid
dilution
(for general lab use)
Indice di rifrazione
n20/D 1.437 (lit.)
P. ebollizione
130-131 °C (lit.)
Punto di fusione
−51 °C (lit.)
Densità
0.951 g/mL at 25 °C (lit.)
Gruppo funzionale
ketone
Stringa SMILE
O=C1CCCC1
InChI
1S/C5H8O/c6-5-3-1-2-4-5/h1-4H2
BGTOWKSIORTVQH-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
It may be used in the following studies:
- Preparation of (2E,5E)-2,5-bis(4-(azidomethyl)benzylidene) cyclopentanone, via cross-aldol condensation.
- Preparation of symmetrical C-5 curcuminoids by reacting with substituted benzaldehyde via Claisen-Schmidt condensation.
- As an electron pair donor to stabilize allyl and vinyl cations during intramolecular carbohydroxylation of alkynes.
Note legali
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2
Codice della classe di stoccaggio
3 - Flammable liquids
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
86.0 °F - closed cup
Punto d’infiammabilità (°C)
30 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
The aldol condensation reaction is an organic reaction introduced by Charles Wurtz, who first prepared the β-hydroxy aldehyde from acetaldehdye in 1872.
The Baeyer-Villiger oxidation is the oxidative cleavage of a carbon-carbon bond adjacent to a carbonyl, which converts the ketones to esters and the cyclic ketones to lactones.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.